Table of Contents
ToggleThe nomenclature of coordination compounds is based on certain rules. These rules are recommended by the International Union of Pure and Applied Chemistry (IUPAC). Thus, names are thus given to the complexes and are called systematic names.
Basic rules of nomenclature of coordination compounds
1. The positive ion comes first, followed by the negative ion.
2. When writing the name of a complex, the ligands are quoted in alphabetical order, regardless of their charge, followed by the metal.
3. When writing the formula of a complex, the complex ion should be enclosed by a square bracket. The metal is written first, then the coordinating groups are listed in the order of negative ligand, neutral ligand, and positive ligands( and alphabetically according to the first symbol within each group).
4. Naming of ligands
The names of negative ligands end in “-O”. Some such ligands are listed in the table below.
| Formula | Name | Formula | Name | Formula | Name |
| F– | Fluoro | O2- | Oxo | N3– | azido |
| Cl– | Choro | O22- | Peroxo | N3- | nitrido |
| Br– | Bromo | HS– | Mercapto | C2O42- | oxalato |
| I– | Iodo | S2- | thio | S2O32- | thiosulphato |
| H– | Hydoido | CN– | Cyano | ONO– | nirito |
| OH– | Hydroxo | SCN– | thiocyanato | NH2– | amido |
| SO4-2 | Sulphato | NO2– | nitro | NH2- | imido |
The neutral ligands are named as such without any special ending.
| Formula | Name | Formula | Name |
| NH2 | ammine | NO | nitrosyl |
| H2O | Aqua | O2 | dioxygen |
| CO | Carbonyl | N2 | dinitrogen |
Positive ligands are named with an ending “-ium.”
e.g: hydrazinium
Thus ligand, through positive can bind through the uncharged nitrogen.
5. Order of ligands
When a complex contains more than one kind of ligand, then, the ligands are listed in alphabetical order. The prefixes (di, tri, and so on) are not considered in choosing, the alphabetical order, numerical prefixes do not affect the alphabetical order.
The prefixes di, tri, tetra, penta, and hexa are used to indicate the number of ligands of the same kind present in the complex.
However, when the name of the ligand itself has a prefix such as di, tri, or tetra (e.g., dipridyl or ethylenediamine) or it is a polydentate ligand (in some cases), then to avoid confusion, Greek prefixes such as bis, tris, and tetrakis are used instead of di, tri, and tetra. In these cases, the name of the ligand is placed in parenthesis (brackets).
6. The naming of isomeric ligands
A type of isomerism among complexes involves the attachment of a ligand to a metal through different donor atoms. For example, the ligand NO2-may be linked to the metal M through N or O. Then, such differences in linkages are indicated by different ligand names. eg,
| Formula | Name |
| M-NO2 | nitro |
| M-ONO | nitrito (linked through O) |
| M-SCN | thocyanato or thicyanato-S |
| M-NCS | isothiocyanate or thiocyano-N |
The oxidation state of the metal is indicated by a Roman numeral in brackets. There should be no spaces between the ligand name and the metal name, and similarly, between the metal name and bracket, the complex ion is considered a single entity.
Complex cations and neutral or molecular complexes have no special endings. But complex anions like [Fe(CN)6]4- should end in -ate. The suffix ate is attached to the name of metal.
| Metal | Ending in the name of the complex anion | Metal | Ending in the name of the complex anion |
| Ag | Argentate | Mn | Manganate |
| Au | Aurate | Sn | Stannate |
| Cr | Chromate | Os | Osmate |
| Co | Cobaltate | Zn | Zincate |
| Cu | Cuprate | Pt | Platinate |
| Fe | Ferrate | Mo | Malybdate |
| Pb | Plumbate | V | Vanadate |
| Ni | Nickelate | Ti | Titanate |
| Hg | Mercurate | Al | Aluminate |
About spacing: Nonionic or molecular complexes are given in a one word name i.e. the name of the neutral complex are given without space. But for the complexes that are ionic, the contains named first and separated from space from the anion.
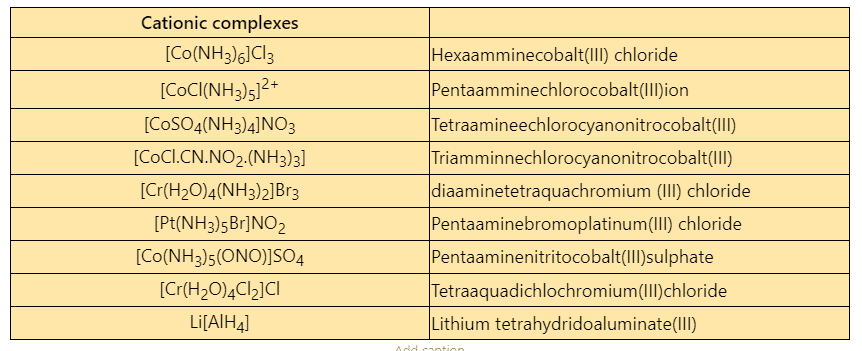
Some examples
FAQs
How to write nomenclature of coordination compound?
The nomenclature of coordination compounds is based on certain rules. These rules are recommended by the International Union of Pure and Applied Chemistry (IUPAC).






