Isomers are chemical compounds with similar chemical formulas but different structural arrangements. Many distinct forms of isomerism exist as a result of the complex formulas of many coordination compounds, the variety of bond types, and the number of possible shapes. Polymerization isomerism, ionization, hydrate linkage, coordination, coordination position, and geometric and optical isomerism are all classified according to Werner’s classification.
Polymerization Isomerism
Certain complexes possess the same stoichiometric composition but different molecular formula compositions. Their actual molecular compositions are multiples of the simplest stoichiometric arrangement. This is not real isomerism because it happens across molecules with the same empirical formula but different molecular weights. Thus [pt(NH3)2Cl2)], [pt(NH3)44[PtCl4), [pt(NH3)4][pt(NH3)Cl3)2 and isomerism may be due to a different number of nuclei in the complex.
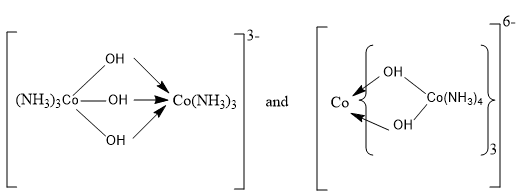
| No. of stoichiometric groups | Platinum series | Cobalt series |
| 1. | [Pt(NH3)2Cl2] | [Co(NH3)3Cl3] |
| 2. | [Pt(NH3)4][PtCl4] | [Co(NH3)6][CoCl6] |
| 3. | [Pt(NH3)4][Pt(NH3)Cl3]2 | [Co(NH3)5Cl][Co(NH3)2Cl4]2 |
| 4. | [Pt(NH3)3Cl]2[PtCl4] | [Co(NH3)6][Co(NH3)2Cl4]3 |
What is polymeriation isomerism?
Certain complexes possess the same stoichiometric composition but different molecular formula compositions, such complexes are said polymerization isomerism.