Table of Contents
ToggleStoichiometric compounds are ones in which the numbers of the various types of atoms or ions present are absolutely in the ratios given by their chemical formulae. They obey the law of constant composition that ‘the same chemical compound always contains the same elements in the same composition by weight.’ Two types of defects may be observed in stoichiometric compounds, called Schottky and Frenkel defects respectively. At absolute zero, the crystal tends to have a perfect arrangement. But, as the temperature increases, the vibration of the ions in their lattice site increase, and ions may jump out of the crystal lattice giving imperfections. These imperfections are known as defects. Schottky and Frenkel defects are two types of defects that can be found in stoichiometric compounds.
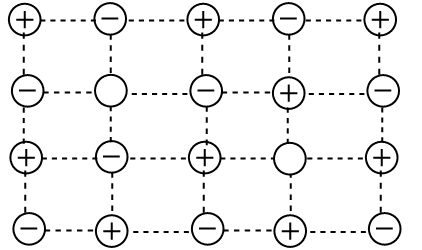
Schottky Defect
A Schottky defect is a form of defect in which a cation and an anion are lost from their normal lattice site, creating a pair of vacancies or holes in a crystal. This type of defect is more prominent in an ionic crystal having a cation and an anion of almost similar size, thus having a high coordination number, generally 6 or 8.
For example, NaCl, KCl, CsCl, KBr, etc.
The number of Schottky defects per cm3 is given by

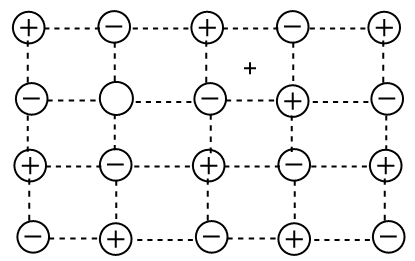
Frenkel Defect
This defect occurs when one ion moves away from its regular lattice site and into an interstitial position. Cations are often smaller than anions. As a result, they are commonly occurring in such positions. Such type of defect is mostly found in crystals in which cations and anions are of different size hence, low coordination numbers 4 to 6.
For example, AgCl, ZnS, CsCl, AgBr, etc.
The number of Frenkel defects per cm3 is given by

Difference Between Schottky defect and Frenkel defect
| Schottky Defect | Frenkel Defect |
| The cation and anion are absent from their lattice position. The number of missing cations and anions is the same. | An ion (cation or anion) departs its lattice position to occupy a vacant interstitial site in the crystal between the cations and anions. |
| The presence of this defect in a crystal decreases its density. | The presence of this defect in a crystal doesn’t change its density. |
| This defect is most common in highly ionic compounds with a high coordination number and cations and anions that are almost the same size. KCl, CsCl, KBr, etc. | The defect generally occurs in crystals having a low coordination number and the cation is smaller in size than anion so that the cation may fit into the interstitial site. For example, AgCl, ZnS, CsCl, AgBr, etc. |






