Table of Contents
ToggleDiagonal relationship is a resemblance of the properties of the elements of 2nd period with diagonally opposite members lying in the 3rd period. The two diagonal elements showing similarities in properties are called diagonal pairs. It is the most important feature of the earlier element of the second period (Li, Be, & B) that they resemble more with the elements lying on the right-hand side in the third-period element (i.e. diagonally opposite element) than with another member of their own group. Thus Li resembles more in its properties with Mg than other alkali metals. Similarly, Be shows similarity with Al, and B resemble Si.
| Period | Group 1 (IA) | Group 2 (IIA) | Group 13 (IIIA) | Group 14 (IVA) |
| 2nd | Li | Be | B | C |
| 3rd | Na | Mg | Al | Si |
The diagonal relationship arises due to resemblance in properties such as atomic size, ionization energy, electronegativity, and polarizing power of their corresponding cations.
Lithium and Magnesium diagonal relationship
The diagonal relationship between Li and Mg is due to:
- Electronegativity of both the elements are almost identical (Li = 1.0, Mg = 1.2).
- Atomic (Li = 1.34 Å, Mg = 1.30 Å) and ionic radii (Li = 0.68 Å, Mg = 0.65 Å) are nearly identical in both elements.
The following points illustrate the diagonal relationship between lithium and magnesium:
- Li and Mg are both harder metals with greater melting points than other metals in their groups.
- When Li and Mg are burnt in the air, they produce the typical oxides Li2O and MgO, which are just sparingly soluble in water.
- On heating, nitrates, carbonates, and hydroxide compounds of both metals decompose in the same way.
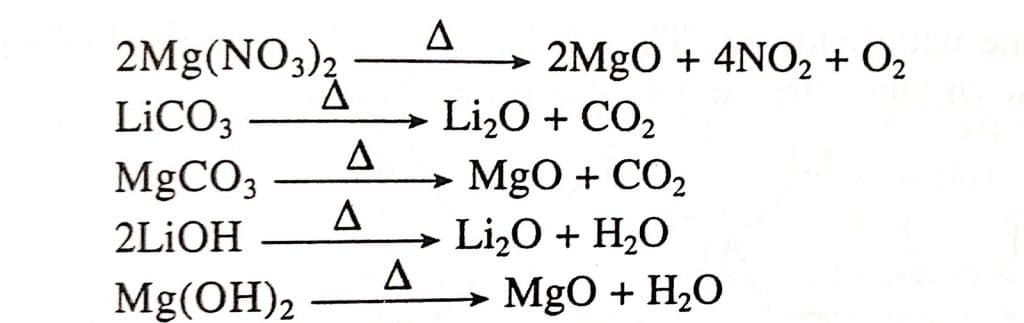
- Both metals react slowly with H2O to form metallic hydroxides, which generate H2 gas.

- Both metal formates form metallic carbonates and formaldehyde when heated.

Diagonal relationship between Beryllium and Aluminium
The diagonal relationship between Be and Al is due to:
- The electronegativity of both elements is almost identical (Be = 1.5, Al = 1.5).
- The covalent radii of both elements are identical.
- Both metals possess a weak electropositive character.
- The ratio between cationic charge and cationic size i.e. Be2+ (0.033) and Al3+ (0.044) ions are almost the same.
The Be and Al diagonal relationship is illustrated by the following points:
- Both metals are typically extracted by electrolytic reduction of their oxides.
- Both metals i.e. Be and Al is present as beryl, 3BeO.Al2O3.6SiO2 in nature.
- Be+2 and Al+3 ions have a great tendency for forming complexes with ligands. Some of the examples are: [Be(H2O)4]++, [Al(H2O)6]+3, [AlF6]-3 and so on.
- In combination with halogens, both the metals behave as Lewis acid. As illustrated below, both BeCl2 and AlCl3 have bridge/dimeric structures.
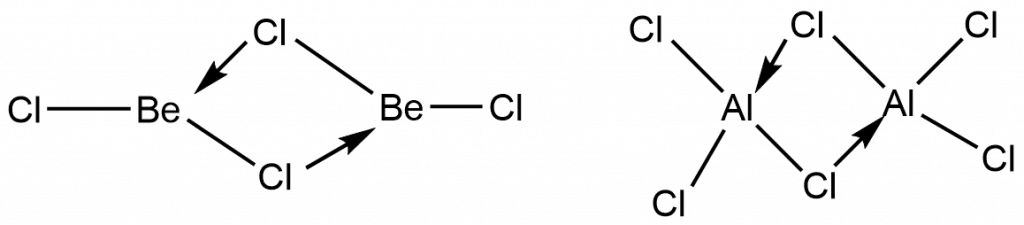
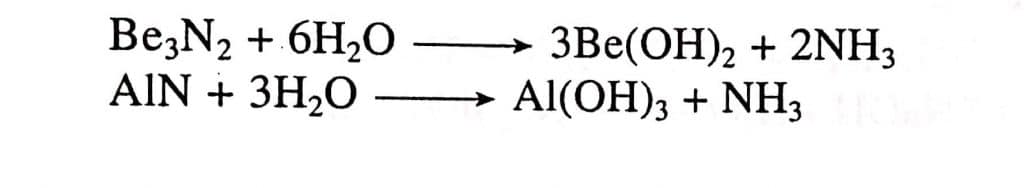
- Both the metals combine with N2 and form their nitrides (Be3N2 and AlN). These nitrides are decomposed by H2O to form metallic hydroxides and ammonia gas is evolved out.
Diagonal relationship between Boron and Silicon
The diagonal relationship between B and Si is due to:
- The electronegativity of both elements is almost identical (B = 2.0, Si = 1.8).
- Both elements have similar cationic charge and cationic size ratio values.
The B and Si diagonal relationship are illustrated by the following points:
- Since both elements are non-metals, they are poor conductors of heat and electricity.
- Most of the compounds of these elements are covalent in nature.
- Both the elements exist in two allotropic forms; amorphous and crystalline. Crystalline forms of both elements are hard.
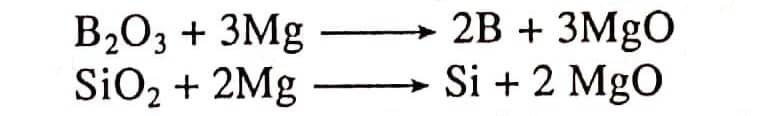
- Both the elements can be obtained by reducing their oxides with Mg metal.
- The oxides of both elements are stable. Because they form boric acid and metasilicic acid in the water, both oxides are weakly acidic in nature.
Diagonal relationship Video
FAQs/MCQs
diagonal relationship definition
Diagonal relationship is a resemblance of the properties of the elements of 2nd period with diagonally opposite members lying in the 3rd period.
diagonal relationship examples
Lithium (Li) and magnesium (Mg), beryllium (Be) and aluminum (Al), boron (B), and silicon (Si); these three pairs show a diagonal relationship.
diagonal relationship is shown by
The diagonal relationship is shown by 2nd (Li, Be, & B) and 3rd (Mg, Al, & Si)-period elements of the periodic table as they possess similar properties.
cause of diagonal relationship
The resemblance in properties such as atomic size, ionization energy, electronegativity, and polarizing power of their corresponding cations are the cause of diagonal relationships.
References
- Rayner-Canham, Geoffrey (22 December 2013). Descriptive inorganic chemistry. Overton, Tina (Sixth ed.). New York
- Shriver, Duward (2006). Inorganic Chemistry (4th ed.).






