Table of Contents
TogglePolarization of ion in the ionic compound induces the covalent character to some extent, this is explained by Fajan’s rule. Before knowing Fajan’s rule, we must know about the polarization phenomenon occurring in the ionic compound. Let’s have the concept of polarization of ion, polarizing power, polarizability as well as the factor affecting them.
Polarization of ion
During the formation of an ionic compound or ionic molecules, two oppositely charged ions ( cations and anions) must come closer to each other. During this process, the cation attracts the electron charge cloud of the outermost shell of the anion toward itself, therefore, the symmetrical shape of the anion gets distorted or deformed, or polarized.
The phenomenon of the distortion of the symmetrical shape of the electron cloud of anion in the nearby cation is called polarization of anion.
For example: In LiI, the electron cloud of I- anion is distorted by cation Li+, which is shown in the following figure.
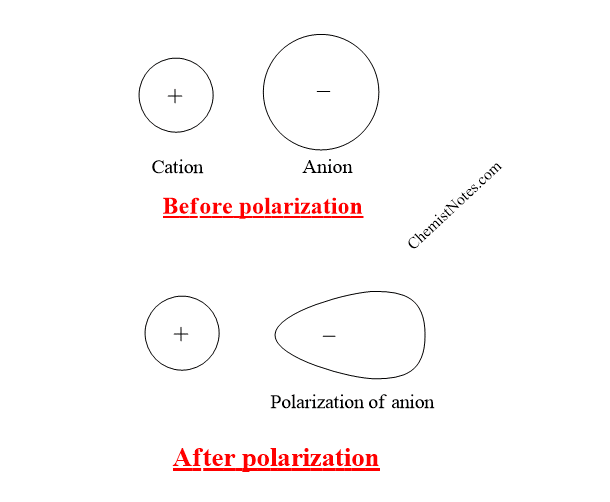
There is also the chance of the polarization of cation by anion but due to the smaller size of the cation, its electrons cloud is strongly held to the nucleus, and therefore, the shape of the cloud is not distorted to an appreciable extent. Hence, the polarization of cation by an anion is not generally considered.
Polarizing power
The ability of a cation to polarize an anion is called its polarizing power or polarizing ability.
Factors affecting polarizing power
The factors affecting the magnitude of polarizing power of a cation are listed below.
- Magnitude of positive charge on the cation
- Size of cation
- Electronic configuration of the cation
Magnitude of positive charge on the cation
The greater the charge on the cation, the more strongly it attracts the outermost shell electron cloud of an anion toward itself and polarizes the given anion easily. Therefore, the polarizing power of cation is directly proportional to the magnitude of the positive charge on it.
Example: Na+< Mg++< Al+++
if the same element has a different positive charge, the higher positive charge has greater power of polarization. For example Sn4+> Sn2+
Size of cation
The smaller the size of the cation, the more strongly it attracts the outermost shell electron cloud of an anion towards itself, and hence, the greater is its polarizing ability. In other words, with the decreasing size of the cation, the polarizing power of the cation increases. Thus, the Polarizing power of the cation is inversely proportional to the size of the cation.
Example: Li+> Na+> K+> Rb+
Electronic configuration of the cation
What if two cations have the same positive charge and almost the same size? In such a case, the electronic configuration of cations determines their polarizing ability. A cation having ns2p6d1-10 electronic configuration will polarize the given anion to a greater extent than the cation having ns2p6 electronic configuration. This can be explained on the basis of the shielding effect of d-electrons. The d-electrons shield the nucleus poorly as compared to the s and p-electrons. Thus, cations having d-electrons have poorer shielding of the nucleus than s- and p-electrons.
Polarizability of anion
The tendency of an anion to get polarized by a cation is called its polarizability.
Factors affecting polarizability
Factors affecting the polarisability of an anion are listed below.
- Magnitude of the negative charges on the anion
- Size of the anion
Magnitude of negative charges on anion
The higher the negative charge on the anion, the more easily its outermost electron cloud is attracted by cations and hence anion is polarized to a greater extent. Thus, the polarisability of an anion is directly proportional to the magnitude of the negative charges on it.
For example:C4-> N3-> O2-> F–
Size of the anion
The larger the size of the anion, the more easily its outermost shell electron cloud is attracted by the cation towards itself, and hence, the greater the polarisability of an anion. Thus, the polarisability of an anion is directly proportional to the size of the anion.
Example: F–<Cl–<Br–<I–






