Table of Contents
ToggleNon-equivalent and equivalent hybridization are the types of hybridization processes that differ from each other in some facts, which are discussed in this post.
Non-equivalent and equivalent hybridization
The three basic s-p hybridizations i.e sp, sp2, and sp3 have the property that in each case, the hybrid atomic orbitals are equivalent. This is true only for these hybridization and such hybrid orbitals are called equivalent hybrid orbitals. But, an intermediate degree of hybridization may occur and all resulting hybrid orbitals are not equivalent such hybrid orbitals are called non-equivalent hybrid orbitals.
Equivalent hybridization
Equivalent hybridization is a type of hybridization in which all hybrid orbitals are of the same shape, size, and energy. All bond angles are the same. Thus, all resulting hybrid orbitals are equivalent and such hybrid orbitals are called equivalent hybrid orbitals.
- It is generally observed when atoms attached to the central carbon are similar. Eg: CH4
- The value of the mixing coefficient (λ) is the same for all hybrid orbitals.
- This hybridization occurs whenever there are s and p electrons with a slight difference in energy or almost equal energy.
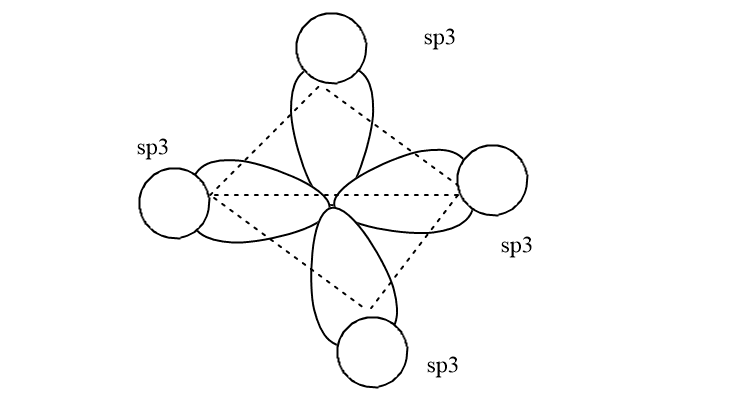
For example: Sp3 hybridization (CH4)
One s and three p orbitals px + py+ pz mix to form four sp3 hybrid orbitals having the same shape ( tetrahedral), size, energy, and bond angle, which is equal to 109o 28′.
Non-equivalent hybridization
Non-equivalent hybridization is a type of hybridization in which all hybrid orbitals don’t have the same shape, size, and energy. Bond angles are also different. Thus, all resulting hybrid orbitals are not equivalent and such hybrid orbitals are called non-equivalent hybrid orbitals.
- It is observed when atoms attached to the central carbon are dissimilar. Eg: CHCl3
- In CHCl3, the values of the mixing coefficient (λ) for hybrid orbitals are not the same. If three of the hybrid orbitals have one value of mixing coefficient and the fourth hybrid orbital will have a different value of λ.
- The hybridization of this kind or type is not only found in carbon. They will occur whenever there are s and p electrons with approximately the same energy.
For example: Molecules of PCl5
Hybridization in this molecule is sp3d. Two types of orbitals geometry are possible in this case.
- Trigonal bipyramidal: In trigonal bipyramidal, two types of hybrid orbitals are formed. Three equivalent orbitals formed from the mixing of s+px + py and the bond angle, in this case, is 120o. Similarly, two equivalent orbitals are formed from Pz + dz2, and the bond angle, in this case, is 90o.
- Square pyramidal: In square pyramidal, two types of hybrid orbitals are formed. Four equivalent orbitals are developed from combination of these orbitals s+ px +py+ dx2-y2. Similarly, One pure pz orbital remained the same.
Non equivalent hybridization youtube video
References
- C. A Coulson, Valence, E.L.B.S., (2nd Edition), 1975
- M. C. Day and J. Selbin, Theoretical Inorganic Chemistry, East-west Press Pvt. Ltd.,1985






