Table of Contents
ToggleRedox titration, also known as oxidation-reduction titration is a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. It is based on an oxidation-reduction reaction between the titrant and the analyte. The standard solutions are either oxidizing or reducing agents. This method involves the use of a redox indicator and/or a potentiometer.
Redox titration is used to determine the concentration of an unknown analyte. The shape of the relevant titration curve must be evaluated in order to analyze redox titrations.
Redox Titration
The oxidation-reduction reaction may simply be represented in general as:
oxidant + ne = reductant
The equilibrium constant for the oxidation-reduction reaction, K, is related to the redox potential E since this reaction involves the transfer of electrons. The potential of the redox system may be expressed in terms of the Nernst equation at 25oC as:
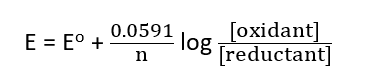
where E and Eo represent the redox potential at any given point during the reaction (titration) and the standard redox potential of the system, respectively, and n represents the number of electrons involved in the reaction. A sudden change in the potential of the two redox systems at the equivalence point provides the means of detecting the endpoint.
By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential that develops in the reaction mixture as a function of the added volume of titrant, the endpoint of a redox titration can be identified. Alternatively, a redox indicator that shows a color change at the endpoint may be used. The progress of the titration and the equivalence point may be computed theoretically in the form of a titration curve.
Requirements For Redox Titration
A redox titration must fulfill the following general requirements:
- The reaction should occur rapidly and to the completion so that the titration can be performed within a reasonable time.
- Between the oxidant and reductant, there should be a definite, well-known equivalent point.
- A suitable technique should be available for the detection of the endpoint.
Redox Titration curve
Let us consider the titration of 100 mL of 0.1M iron(II) with 0.1M Cerium(IV) in the presence of dil. H2SO4:
Ce+4 + Fe2+ ⇌ Ce+3 + Fe+3
The quantity corresponding to [H+] in acid-base titration is the ratio [ox]/[red].
There are two systems:
Fe3+ / Fe2+ ion electrode (1), & Ce4+ / Ce3+ ion electrode (2)
For (1) at 25oC, the redox electrode potential is

For (2) at 25oC, the redox electrode potential is

The equilibrium constant of the reaction is given by
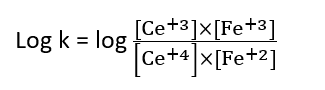
During the addition of the Ce(IV) solution up to the equivalence point, its only effect will be to oxidize iron(II) and consequently change the ratio [Fe3+] / [Fe2+]. When 10 mL of the oxidizing agent has been added,
[Fe3+] / [Fe2+] = ~10/90 (approx) and
E1 = 0.75 + 0.0591 log10/90 = 0.75-0.056 = 0.69 volt
With 50 mL of the oxidizing agent: E1 = E1o = 0.75
With 90 mL, E1 = 0.75 + 0.0591 log90/10 = 0.81 volt
At the equivalent point (100 mL) [Fe3+ ] = [Ce3+] and [Ce4+] = [Fe2+ ]
And the electrode potential is given by:

The subsequent addition of Cerium (IV) solution will merely increase the ratio [Ce4+] / [Ce3+].
Thus, with 100.1 mL, E2 = 1.45 + 0.0591log0.1/100 = 1.27 volts
Similarly, with 190 mL, E2 = 1.45 + 0.0591 log90/100 = 1.45
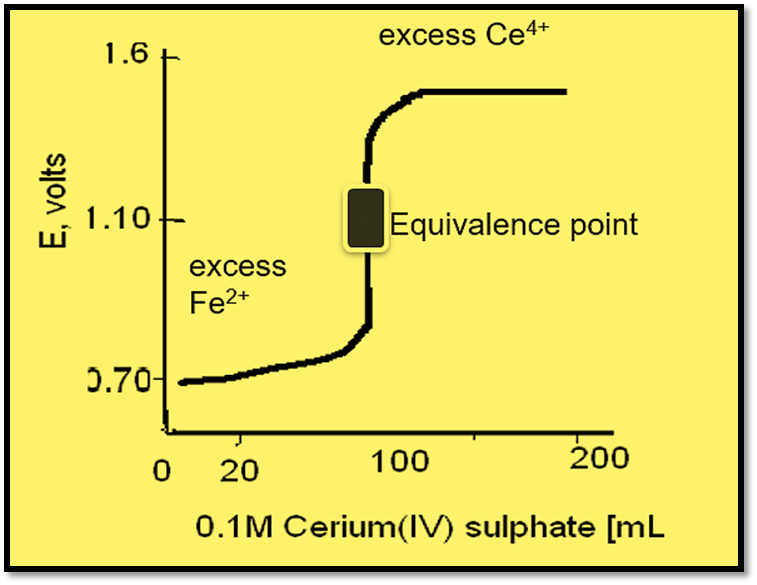
The titration curve shows that there is a sudden sharp change of redox potential at the neighborhood of the equivalence point. Hence suitable redox indicator can be used to detect the equivalence point of redox reaction by titration.
Redox Indicators
Redox indicators are organic compounds that can be oxidized or reduced and they exhibit different colors in their oxidized and reduced forms.
Inox + ne ↔ In red
Diphenylamine (0.76 V), Methylene blue (0.52 V), N- Phenylanthranilic acid (0.89 V), Ferroin ( 1, 10- phenanthroline iron (II) sulphate, (1.06V), Nitro ferroin (5- nitro-1, 10-phenanthroline iron(II) sulphate, (1.25 V), etc. are some commonly employed redox indicators.
Characteristics of redox indicators
- A redox indicator should mark the sudden change in oxidation potential near the equivalence point in a redox titration.
- The oxidation and reduction should be reversible and quick.
- The ideal redox indicator will be one with an oxidation potential intermediate between that of the solution titrating and that of the titrant and which exhibits a sharp readily detectable color change.
- Each redox indicator changes color over a particular potential range.
- For a sharp color change at the endpoint, the standard potential of the indicator should differ by at least 0.15V from the standard potential of the redox system.
Mechanism of redox indicator
One of the first redox indicators that were frequently employed in the titrimetric analysis was diphenylamine. The barium or sodium salt of diphenylamine sulfonic acid is more frequently utilized since this compound is not easily soluble in water and because tungstate ion and mercury(II) chloride interfere with its activity. This indicator comes in reduced and oxidized forms, the latter of which has a deep violet color. With the use of diphenylamine, as an illustration, the mechanism underlying the color change has been demonstrated as:
The solution of diphenylamine in Conc. H2SO4 is colorless. It is employed in the titration of Fe(II) with K2Cr2O7 solution. It is initially converted to colorless diphenyl benzidine using an oxidizing agent. This is then further reversibly oxidized to produce diphenyl benzidine violet.
If diphenyl benzidine violet is let to stand in an excessive dichromate solution, it will further oxidize. The further oxidation is irreversible, and compounds that are red or yellow and have an unknown composition are formed.
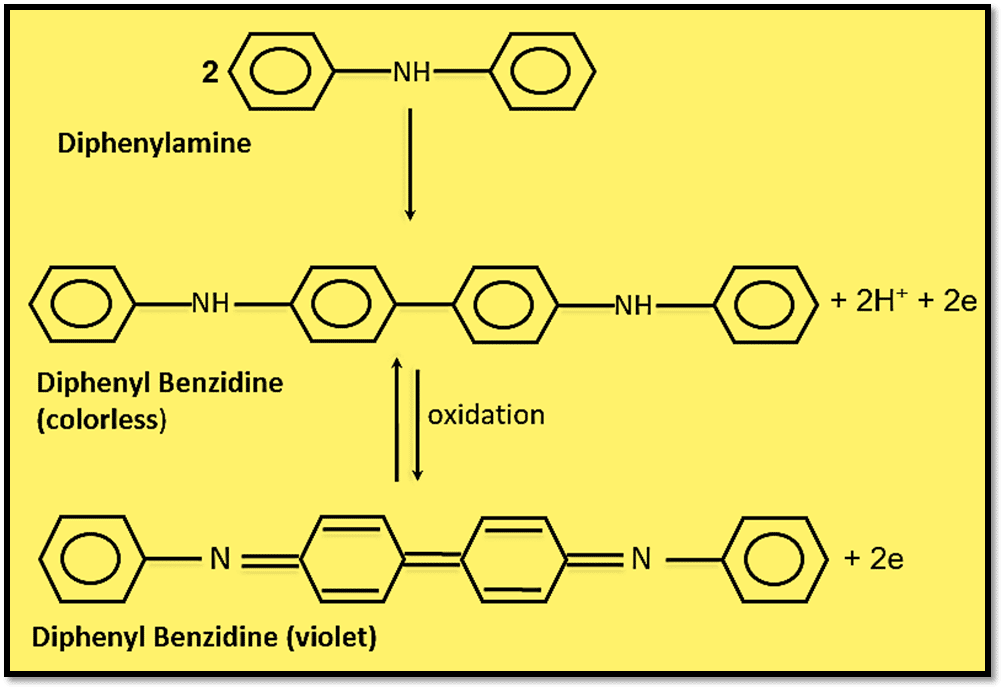
Moreover, the presence of a long conjugated system in the diphenyl benzidine leads to the absorption of light in the visible region, and hence it is colored.
Application of redox titrations
- Determination of the concentration of an unknown analyte.
- Evaluation of the COD (chemical oxygen demand) of wastewaters, and natural waters.
- To check the purity of raw materials such as evaluating the chlorination of public water supplies.
- The quantification of valganciclovir hydrochloride (VLGH) in pure drugs and capsules uses redox titration in pharmaceutical analysis.
- In the food industry; analyze if a product contains salt, sugar, or a high concentration of vitamin C or E, which can affect the color of the product.