Table of Contents
ToggleIon selective electrode is a widely used analytical tool for sensing ions in various real samples of clinical, industrial, environmental, and laboratory research. These are membrane electrode, that acts as a sensor, that converts the activity of a specific ion into an electrical potential.
What is an ion selective electrode?
Ion selective electrodes are the electrode that generates potential in the response to the presence of a solution of a specific ion. Since this electrode detects specific ions only, it can also be considered a transducer or sensor. These are also called specific ion electrodes (SIE).
As mentioned already, these convert the activity of a specific ion dissolved in a solution into an electrical potential.
Ion selective electrode principle
An ideal ISE consists of a thin membrane across which only the specific ion can be transported. The transport of ions from a high concentration to a low concentration one through selective binding with some sites within the membrane creates a potential difference, this is the fundamental principle of ISE.
Types of Ion selective electrode
On the basis of membrane materials, Ion selective electrodes can be classified into the following major types.
- Glass membrane electrode
- Solid state membrane electrodes
- Liquid membrane electrode
- Gas sensing electrode
Glass membrane electrode
Glass membrane electrodes are used for sensing univalent cations such as H+, Na+etc. These electrodes are formed by doping molten SiO2 with various chemicals. Thus, different glass compositions can be made to measure Na+, K+, Ag+, NH4+, etc. In other words, the selectivity for these cations is achieved by varying the composition of a thin ion-selective glass membrane.
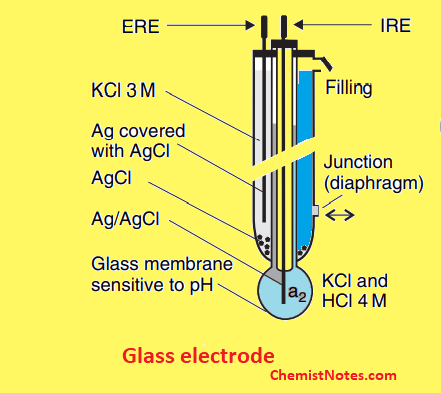
Examples of glass membrane electrodes include H+ sensitive electrodes or PH electrodes.
When the glass electrode is dipped into the solution, a rapid equilibrium establishes across the glass membrane with respect to the hydrogen ions in the inner and outer solutions, producing a potential.
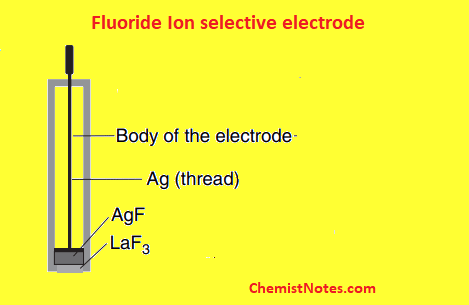
Solid state membrane electrode
Solid-state ionic conducting membranes are used in these electrodes. These membranes are made from ionic compounds or a mixture of ionic compounds.
One of the common examples of such electrodes is the fluoride ion selective electrode. This consists of LaF3 crystal and an internal electrolyte solution consisting of 0.1M NaF and 0.1M KCl and containing the Ag/AgCl wire.
The LaF3 crystal is doped with EuF2 to provide vacancies(holes) at anionic sites. Since each EuF2 introduces only two F– instead of three, there is a vacant fluoride site for each EuF2 added. Thus, F– from solution can moves by migration through the crystal lattice from one vacancy defect to another and establish the desired potential difference.
Liquid membrane electrode
The liquid membrane electrode consists of two concentric tubes in which the inner tube contains an aqueous reference solution and an internal reference Ag/AgCl electrode. The outer tube contains a reservoir of organic ion exchangers as shown in the example.
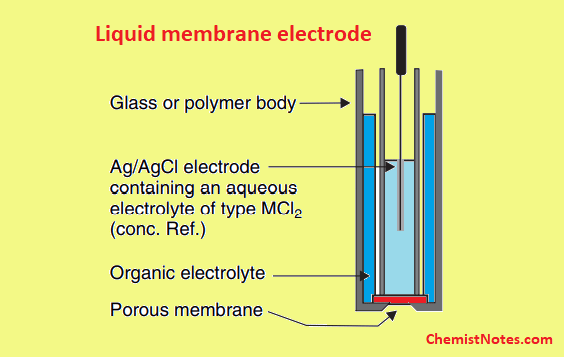
The separation between internal and external solutions of the electrode is achieved by using a porous hydrophobic membrane or disc. The electrode’s ion selectivity can be changed by altering the membrane.
A calcium ion selective electrode is one example of such type of electrode. This electrode uses calcium salts of bis-(2-ethyl hexyl) phosphoric acid dissolved in straight chain alcohol as an ion exchanger membrane. At the interface of membrane/sample solution, such membrane is capable of exchanging the Ca++ ions of sample solution. A measurable potential difference will appear if the concentrations of the calcium ions are different on the two sides of the membrane.
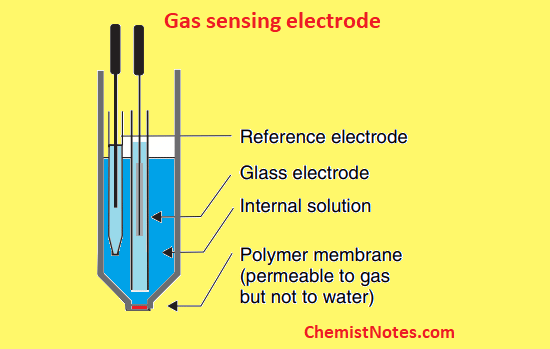
Gas sensing electrode
An ammonium ion selective electrode is an example of an ion-selective electrode. These electrodes have been devolved to measure dissolved gas such as carbon dioxide, ammonia, sulfur dioxide, etc.
These chemical sensors consist of an internal solution that is separated from the sample solution(test solution) by a hydrophobic membrane that is permeable to dissolved gas molecules. Such an electrode includes a glass electrode in contact with a bicarbonate solution of low concentration (0.01M). A polymer membrane separates the bicarbonate solution from the sample solution, allowing the gas analyte to diffuse towards the inner electrode compartment.
Applications of Ion Selective electrode
Ion selective electrodes can be used:
- To determine various ions in aqueous solutions.
- To measure the pH of the solution.
- To monitor pollutants in natural water and effluents by measuring CN–, F–, S–, Cl–, NO3– etc.
- To measure different types of ions present in the soil.
- To measure NO3–, NO2– ions in meat preservatives, and salt content in meat, fish, and fruit juices.
- To determine fluoride ions in drinking water and other drinks.
- To determine ions such as Ca++, K+, Cl– etc in body fluids like plasma, serum, and sweat.
- As a chemical sensor for research purposes.
Advantages of ion selective electrode
- Simple, low-cost, and easy to handle.
- It can be miniature, and non-destructive.
- It can be used for the analysis of a continuous flow system.
Limitations of ion selective electrode
- The detection limit is not good as other techniques.
- There may be problems due to interferences.
- It can’t be used for a long time due to the deterioration of the membrane.






