Table of Contents
ToggleElectron impact ionization in mass spectrometry often leads to significant fragmentation of molecules, making it challenging to observe intact molecular ions. To address this issue, an indirect ionization method known as chemical ionization(CI) is used. This technique was first introduced by Munson and Field in 1966.
What is Chemical ionization?
Chemical ionization is the technique of ionization in which the vaporized sample is inserted into the mass spectrometer accompanied by an excess of reagent gas at around 1 torr pressure. Commonly methane, ammonia, and isobutane are used as a reagent. By electron interaction with the main ions CH4+. and CH3+, the excess carrier gas is ionized and the secondary ions are produced when these interact with the excess methane.
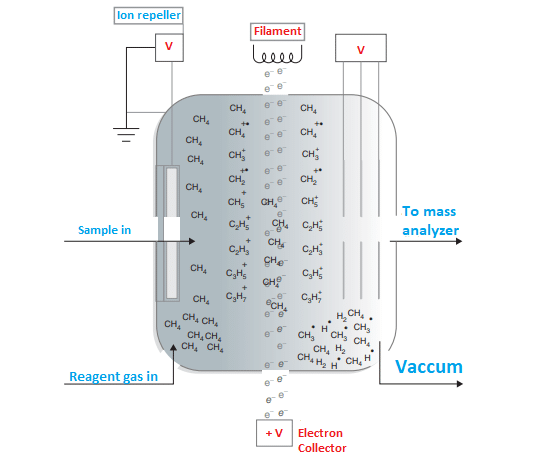
Principle of Chemical Ionization
In chemical ionization, the high-energy electron beam does not directly bombard the sample molecules in the vapor phase. Instead, a reagent gas such as methane, isobutane, ammonia, etc is ionized at an ionization source. These ionized reagent gas molecules collide with sample molecules at relatively high pressure, thus sample molecules undergo ionization.
The ionized reagent gas also known as secondary ions reacts with the sample(M).
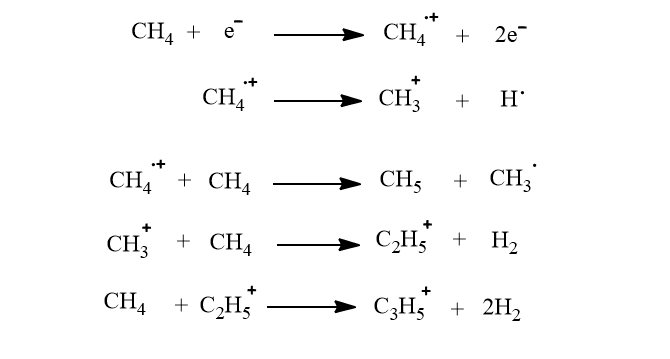
Since less than 5 eV energy is transferred to the sample molecule by ionized reagent gas during collision much less fragmentation takes place.
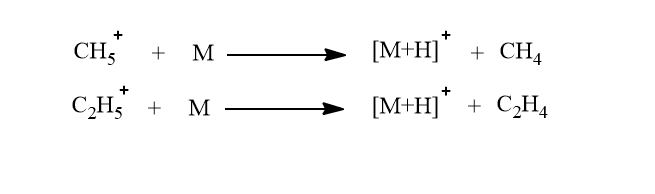
This [M+H]+ ion is generally known as a quasimolecular ion and these are mostly observed as intense peaks in the mass spectra.
Example: Chemical ionization of 3,4-dimethoxy acetophenone
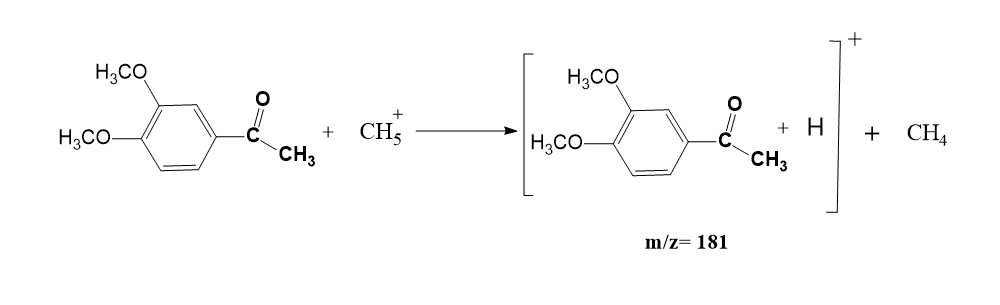
Note: Chemical ionization can be carried out in the following different modes.
- Positive-ion CI
- Negative-ion CI
- Electron-capture negative ionization (ECNI)
Chemical ionization Vs Electron impact ionization
Chemical ionization is simply a soft ionization technique that provides an advantage over electron impact ionization as electron impact ionization often produces extensive fragments such that the parent ion is not observed whereas high energy electrons are not used to bombard chemical ionization. When compared to the EI spectra, the CI spectra revealed a greater abundance of ions in the [M+H]+ in the molecular weight area, which helped to determine and identify these compounds’ molecular mass.
Advantages of Chemical Ionization
- This soft ionization technique results in reduced fragmentation and detection of molecular ion peaks.
- Simply resolution and determination of the accurate molecular weight of the analyte can be achieved.
- By selecting the proper reagent ion, the selectivity of chemical ionization may be readily enhanced.
- Obtains chemical fingerprint data for samples for chemometric analysis.
- It can measure atmospheric positive and negative ions, sulfur compounds, and hydrocarbons.
Disadvantages of Chemical ionization
- The sample must be volatile and stable.
- This technique is unsuitable for non-polar and thermally labile compounds.
- Generally used for low-mass compounds (<100 Da)
- Little fragmentation and hence no fragment library.
- Limited structural information.
- The selection of reagent gas is complex because different reagent gases can produce varying ionization reactions and selectivity towards specific analytes.
Application of Chemical Ionization
- It is used for the determination of nonpolar, volatile compounds in different fields such as environment, food safety, or toxicology.
- It can be used in the determination of melamine in milk powder.
- Determination and identification of the quality of tea
- Since various tea kinds contain varying amounts of the same chemical, different teas have distinct mass spectra when detected by CI. The chemical fingerprint properties of the tea can be somewhat reflected in the mass spectrum. As a result, CI offers significant practical application for the quick identification and quality assessment of the tea.
- It has applicability in the rapid detection of trace quantities of analytes in pharmaceutical preparations.
- It has been used for continuous monitoring of atmospheric OH and H2SO4.
- Detection of potential perfluorooctane sulfonate precursors and fluorotelomer alcohols through environmental sampling using chemical ionization which in turn will help to determine the distribution of fluorinated organic contaminants that is affecting the health of humans and animals.
- It can be applied in the analysis of hydrocarbon and petroleum products in petrochemical industries.
- It has gained its applicability in natural product chemistry as well. It helps to identify and characterize certain classes of compounds such as alkaloids, flavonoids, and terpenoids.
Published by: Soniya Joshi
Chemical ionization video
References
- Martin, J. W.; Muir, D. C. G.; Moody, C. A.; Ellis, D. A.; Kwan, W. C.; Solomon, K. R.; Mabury, S. A. Collection of Airborne Fluorinated Organics and Analysis by Gas Chromatography/Chemical Ionization Mass Spectrometry. Anal. Chem. 2002, 74 (3), 584–590. https://doi.org/10.1021/ac015630d.
- Berresheim, H.; Elste, T.; Plass-Dülmer, C.; Eiseleb, F. L.; Tannerb, D. J. Chemical Ionization Mass Spectrometer for Long-Term Measurements of Atmospheric OH and H2SO4. International Journal of Mass Spectrometry 2000, 202 (1), 91–109. https://doi.org/10.1016/S1387-3806(00)00233-5.
- Munson, B. Chemical Ionization Mass Spectrometry: Theory and Applications. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd, 2006. https://doi.org/10.1002/9780470027318.a6004.
- Silverstein, R. M.; Bassler, G. C. Spectrometric Identification of Organic Compounds. J. Chem. Educ. 1962, 39 (11), 546. https://doi.org/10.1021/ed039p546.






