Table of Contents
ToggleAlkaloids are nitrogen-containing chemical compounds derived from plants and animals. These are regarded as a large group of compounds possessing biological, pharmacological, physiological, and chemical activities. Many natural alkaloids including various derivatives have already been created as medicines to treat a variety of ailments.
What are alkaloids?
Alkaloids can be defined as naturally occurring large groups of pharmacologically active nitrogen-containing secondary metabolites present in plants, microbial, or animals.
The term alkaloid was derived from alkali and the Greek word oeides which means like. It means these are water-soluble bases.
Most of the time, nitrogen remains as a part of one or more rings but there are instances in which it exists the rings and becomes a component of a side chain as an amino or substituted amino group. Muscaline, choline, and betaines are common examples in which nitrogen is present as a side chain.
Characteristics of alkaloids
The major chemical properties of alkaloids have been described below.
- They are mainly plant-derived and are solid in form however, some are liquid as well. Nicotine and cotinine are found in the liquid state.
- They are highly reactive chemicals with biological activity.
- They taste bitter and are solid white, with the exception of nicotine which is brown in color.
- They are mostly crystalline in nature.
- They are basic in nature due to the presence of lone pairs of electrons on the nitrogen atom.
- They react with acids to form salts, showing basicity.
- They may occur in plants in the free state or as salts and as N-oxides.
Chemical test for alkaloids
Alkaloids present in various samples (crude extract) can be detected in the laboratory by using simple chemical tests. 6 chemical tests for alkaloids have been listed below:
Mayer’s test
Potassiomercuric iodide solution is known as Mayer’s reagent. 1.3 grams of Mercuric chloride and 5 grams of potassium iodide are dissolved in 100mL water to prepare this reagent.
In this test, about 1 mL sample solution(containing alkaloids) is mixed with 1 mL of Mayer’s reagent and then shaken to mix. The appearance of a cream precipitate indicates the presence of alkaloids.
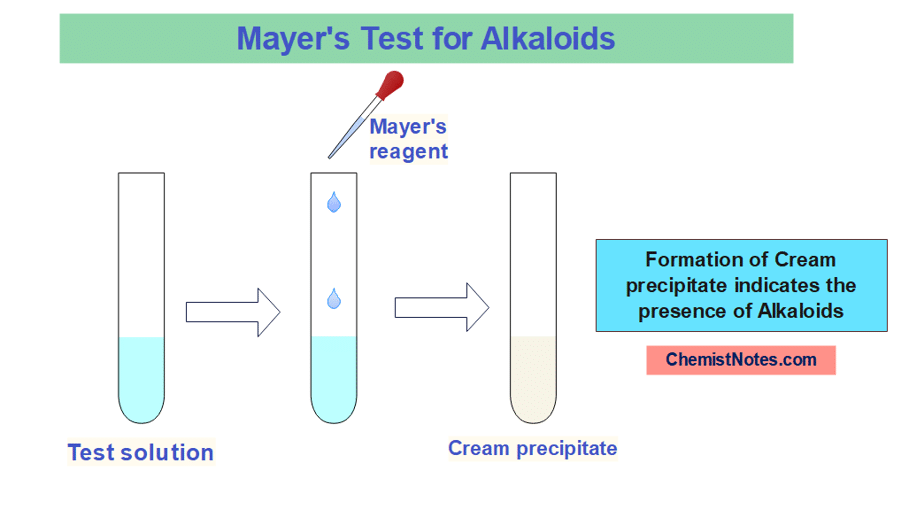
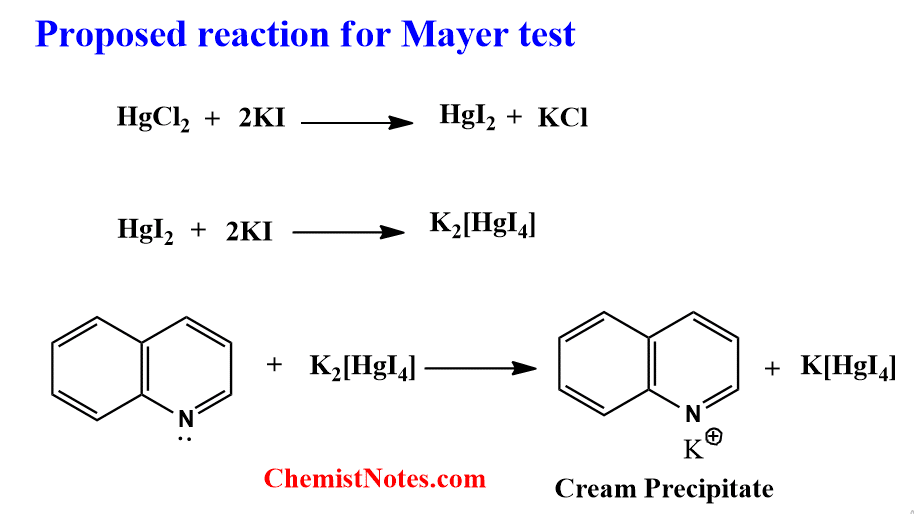
The lone pair of electron present in the nitrogen atom of alkaloid forms a coordinate covalent bond with the Potassium metal ion, thus cream colored complex is formed as a precipitate.
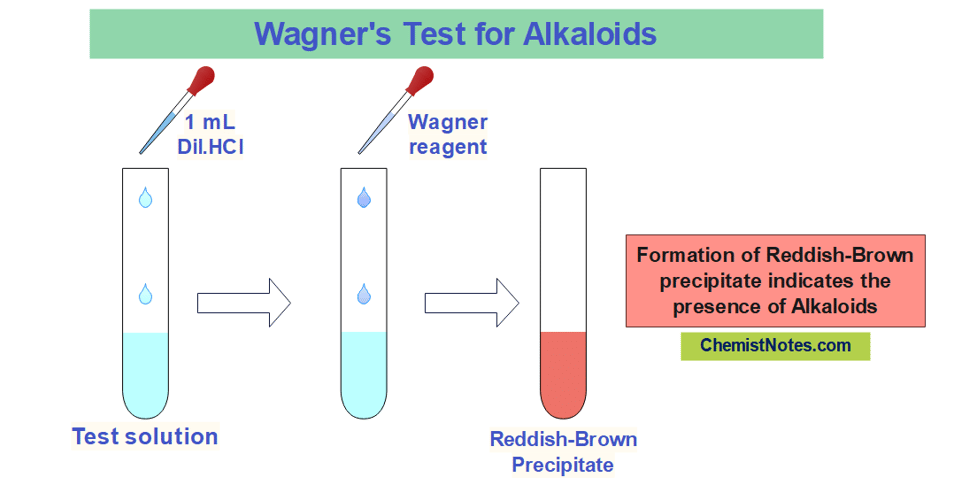
Wagner’s test
The iodine in Potassium iodide solution is commonly called a Wagner reagent. The chemical name of the Wagner reagent is iodo-potassium iodide. 1 gram of iodine and 3 grams of Potassium iodide are dissolved in 50mL distilled water to prepare this reagent.
In this test, about 2-3 mL of the sample solution is mixed with 1 mL of dilute HCl followed by a few drops of Wagner’s reagent. The appearance of a reddish-brown precipitate indicates the presence of alkaloids.
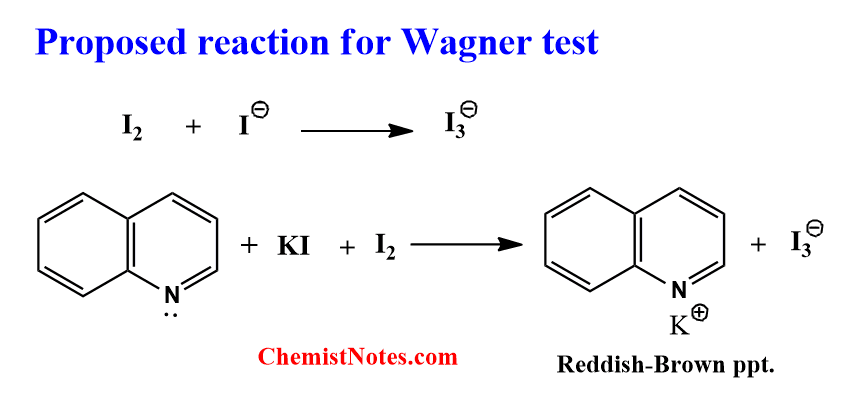
During the preparation of the reagent, iodine(I2) reacts with KI to form I3– which has a brown color. The potassium ion forms a coordinate covalent bond with the nitrogen of the alkaloid forming a complex reddish-brown precipitate.
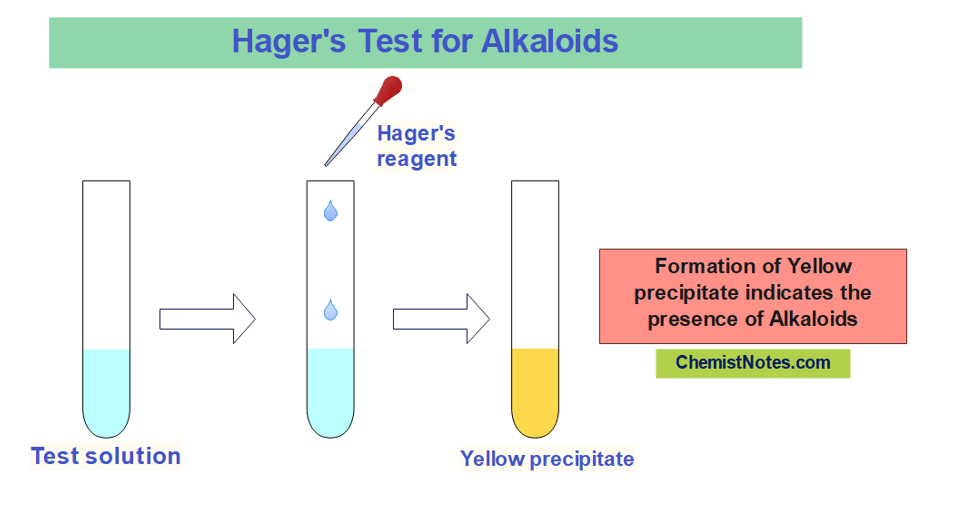
Hager’s test
A saturated solution of Picric acid is known as Hager’s reagent. 1 gram of picric acid is dissolved in 100 mL of distilled water to prepare this reagent.
In this test, about 1 mL of test solution is mixed with a few drops of Hager’s reagent. The appearance of a yellow precipitate indicates the presence of alkaloids.
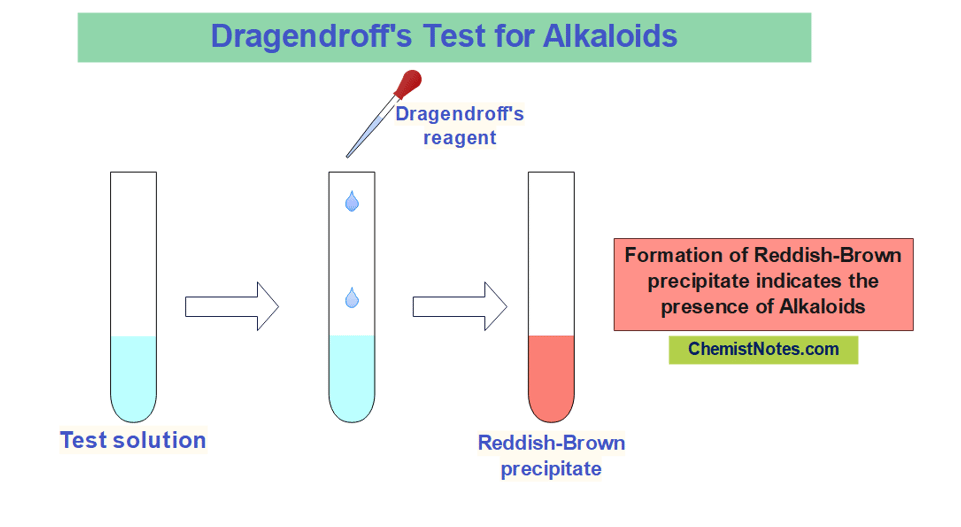
Dragendroff’s test
A solution of Potassium bismuth iodide is commonly known as Dragendroff’s reagent(DR) or, iodobismuthate reagent. Bismuth nitrate is dissolved in diluted acid (acetic or tartaric acid, very seldom hydrochloric or sulfuric acid), and then combined with a strong solution of potassium iodide to create DR. For this reagent, a low pH is necessary.
0.85 grams of basic bismuth nitrate is dissolved in 40 mL of distilled water and 10 mL acetic acid to prepare Solution A. Similarly, 2 grams of Potassium iodide is dissolved in 20 mL of distilled water to prepare Solution B. 100 mL of distilled water and 20 mL acetic acid solution is then added to 10 mL of each of solution A and B. Then after, equal parts of the solution A and B are mixed to prepare Dragendroff’r reagent.
In this test, the test solution is treated with a few mL of Dragendroff’s reagent. The appearance of an orange or reddish-brown precipitate indicates the presence of Alkaloids. However, such a colored precipitate is not formed for Caffeine and other purine alkaloids.
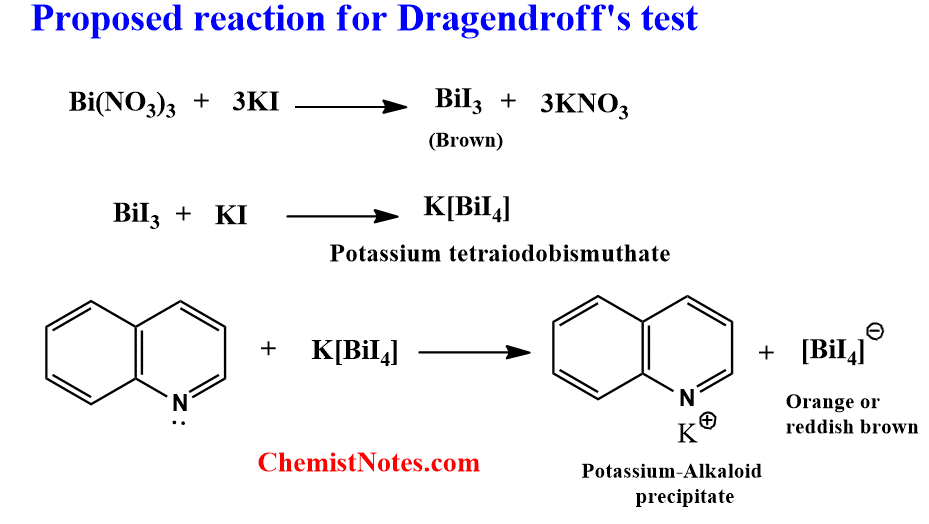
The proposed reaction for Dragendroff’s test is shown below:
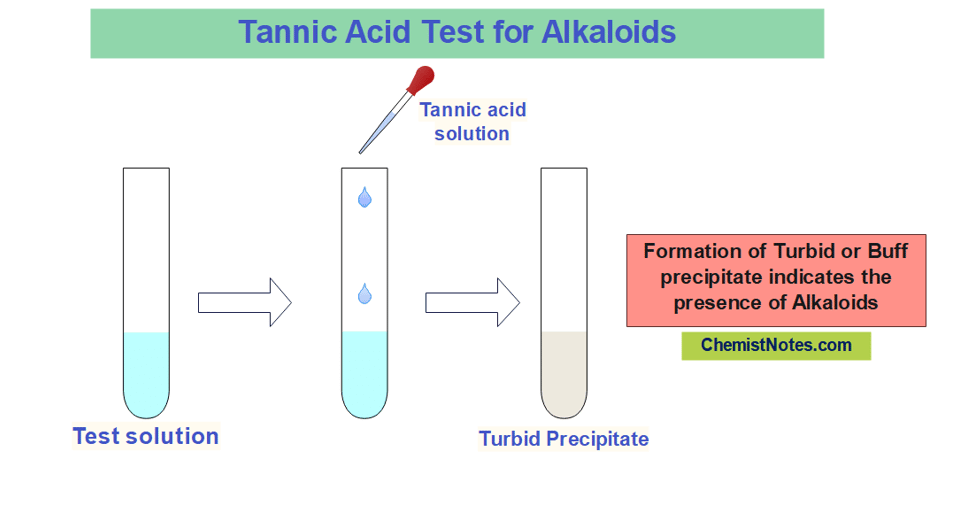
Tannic acid test
5 grams of tannic acid is dissolved into 100 mL of distilled water to prepare the tannic acid solution for the test of alkaloids. In this test, the test sample is treated with a few mL of tannic acid solution. The appearance of turbid or buff precipitate indicates the presence of alkaloids.
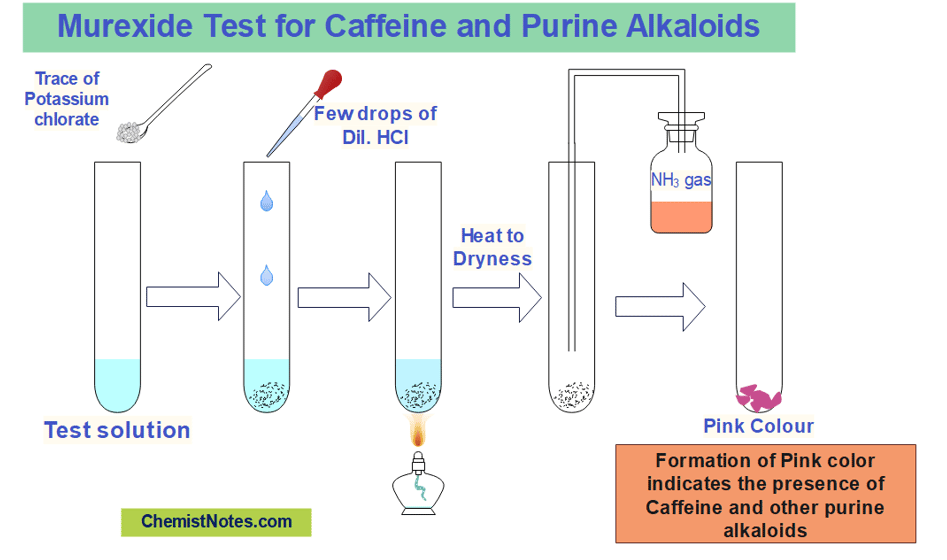
Murexide test
This is a special test for Caffeine and other purine alkaloids since these can not be tested by Dragendroff’s test.
In this test, a little amount of potassium chlorate and a drop of hydrochloric acid are added to the test sample followed by evaporation to dryness, and the resultant residue is then subjected to ammonia vapor. The appearance of pink color indicates the presence of Caffeine and purine derivatives.
Classification of alkaloids
As we have mentioned earlier, alkaloids are secondary metabolites and these can be classified on the basis of their (1) biological and ecological activity (2) chemical structure(heterocyclic rings) (3) biosynthetic pathways.
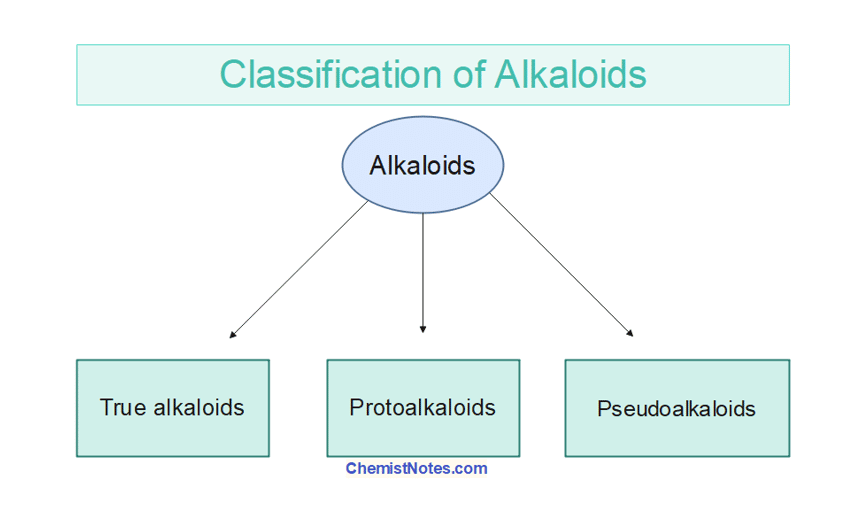
Classification on the basis of Amino acid precursor
Alkaloids can also be classified into the following three types on the basis of whether these are derived from amino acids or not.
- True alkaloids
- Protoalkaloids
- Pseudoalkaloids
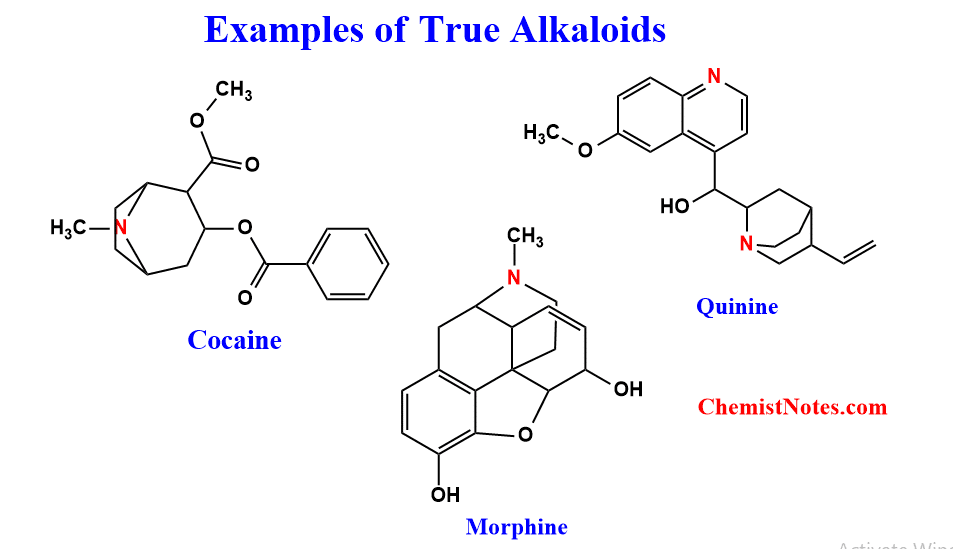
True alkaloid
These alkaloids are derived from amino acids and they have nitrogen present in the heterocyclic ring. The primary precursors of true alkaloids are amino acids such as L-ornithine, L-Lysine, L-phenylalanine, L-Tryrosine, L-tryptophan, L-histidine, etc. Examples of True alkaloids are Cocaine, quinine, dopamine, morphine, usamberensine, etc.
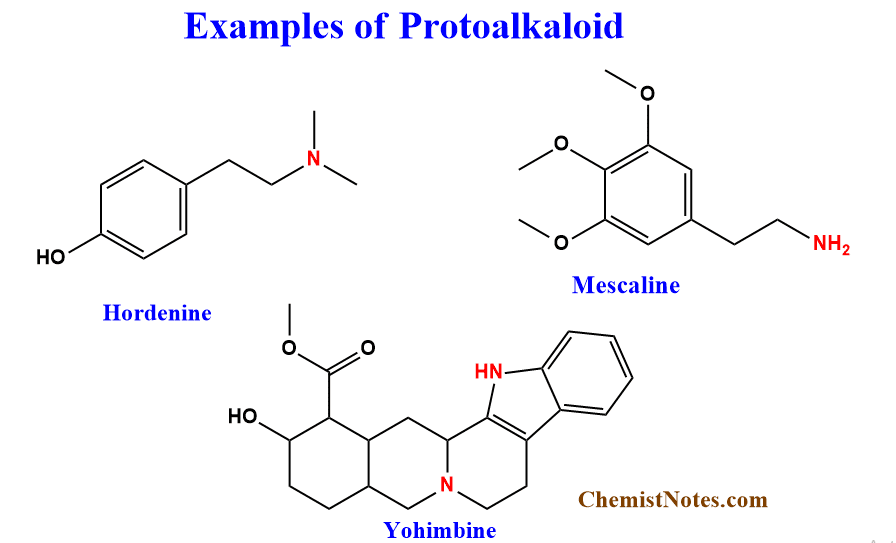
Protoalkaloid
These alkaloids are chemical compounds in which the N-atom derived from amino acids is not a part of heterocyclic. These are mostly derived from L-tyrosine and L-tryptophan and L-ornithine. Examples of Protoalkaloids include Hordenine, Mescaline, yohimbine, etc.
Pseudoalkaloid
These are compounds having basic carbon skeletons not derived from amino acids. Although pseudoalkaloids are connected with amino acid pathways, the basic carbon skeleton is not derived from amino acids. These can also be derived from non-amino acid precursors. The N-atoms are inserted into the molecules at a relatively late stage. Examples include Capsaicin, Ephedrine, Solanidine, Caffeine, Theobromine, etc.
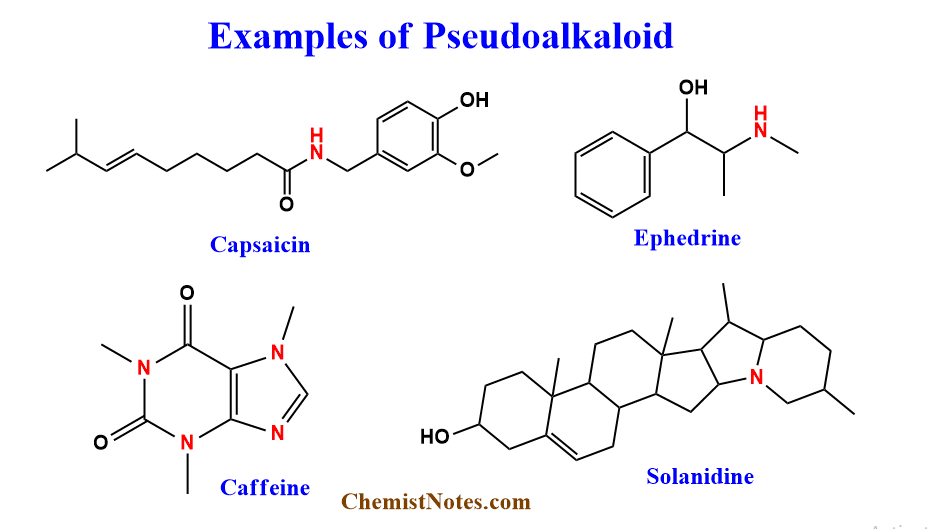
Classification on the basis of heterocyclic rings(Chemical structure)
Similarly, alkaloids are classified into various according to the heterocyclic rings they contain or on the basis of the heterocyclic nuclei.
- Pyrrolidine alkaloid: Hygrine, Nicotine etc.
- Tropane alkaloid: Atropine, Cocaine, Ecgonine, etc.
- Pyrrolizidine alkaloid: Echimidine and symphitine
- Piperidine alkaloid: Piperine, Sparteine, Trigonelline, etc.
- Quinolizidine alkaloid: Cytisine, Lupanine, etc.
- Indolizidine alkaloid: Swainsonine and Castanospermine.
- Phenylethyl-amino alkaloid: Dopamine, Adrenaline, etc.
- Phenethyl isoquinoline alkaloid: Galanthine, Galanthamine, etc.
- Indole alkaloid: Serotonin, Arundacine, etc.
- Quinoline alkaloid: Chinchonine, Brucine, Quinine, Quinidine
- Pyroloindole alkaloid: Corynanthine, Chimonantheine, etc.
- Ergot alkaloid: Ergobine, Ergotamine, Ergocryptine, etc.
- Imidazole alkaloid: Pilocarpine
- Manzamine alkaloid: Xestomanzamine A, Xestomanzamine B, etc.
- Marine alkaloid: Saxitoxin,Tetrodotoxin.
- Quinazoline alkaloid: Peganine.
- Isoquinoline alkaloid: Codeine, berberine, morphine, etc.
- Acridine alkaloid: Acronycine, Rutacridone, etc.
- Terpenoid indole alkaloid: Yohimbine
- Piperidine alkaloid: Coniine, Coniceine, Pinidine, etc.
- Sesquiterpene alkaloid: Cassinine,Celapanin, Evonine, etc.
- Ephedra alkaloids: Cathine, Cathinone, Ephedrine, etc.
- Aromatic alkaloid: Capsaicin.
- Terpenoid alkaloid: Aconitine.
- Steroidal alkaloids: Batrachotoxin, conanine, Solanine, etc.
- Purine alkaloid: Caffeine, theobromine, and theophylline
Health benefits of Alkaloids
- Numerous alkaloids including Caffeine, Cathine, Theobromine, and Theophylline are known to have stimulating effects.
- It is believed that caffeine increases analgesic action because it affects the central adenosine receptor. Additionally, it works effectively to treat some types of headaches and dermatitis.
- Both caffeine and theobromine which are used in medicine, stimulate the central nervous system and boost blood flow.
- Since it may be used as a relaxant and to avoid bronchospasms, theophylline has applications in medicine.
- There is evidence that several alkaloids have antimicrobial properties. As berberine inhibits HIV I reverse transcriptase, alkaloids like it may be utilized to treat AIDS.
- Alkaloids with immunosuppressive properties include Vinblastine and Vincristine. These are also well-known anticancer medications. These have been utilized to produce therapeutic drugs for the treatment of cerebral and pulmonary problems as well as Hodgkin’s disease, tumors, and blood disorders.
- Yohimbine inhibits presynaptic α2 adrenoreceptors and causes sympathetic nerve terminals to release more noradrenaline. It can be used to raise blood pressure and heart rate as well as to treat various forms of male impotence.
- Although strychmine is one of the most lethal alkaloids to animals, its derivatives are utilized to treat eye and optic nerve disorders.
- Some are used as metabolism stimulants and to alleviate gastrointestinal problems. Boldine is one example.
- Galanthamine is used to treat Alzheimer’s disease because it inhibits the action of the enzyme acetylcholine esterase.
- As a muscle relaxant, tubocurarine is utilized in surgical procedures. Similar to nicotine, lobeline is similarly effective in treating vascular problems.
- Some alkaloids including aconitine, control the activity of the Na+ channels, which in turn affects receptor activity and enzymatic activity controlled by the receptor. It can thus be utilized to treat abnormalities in local and neuronal signaling as well as nerve activity at receptors.
FAQs/MCQs:
Alkaloids in Coffee
Caffeine and Trigonelline are two major alkaloids present in Coffee.
References
- Swan, G. A. 1967. An Introduction to the Alkaloids. New York: Wiley.https://doi.org/10.1002/ange.19680801548
- Small, L. F. 1943. Alkaloids. In: Organic Chemistry. An Advanced Treatise. Second Edition. Vol. 2 (Gilman, H., ed.), pp. 1166–1171
- Cordell, G. A. 1981. Introduction to Alkaloids. A Biogenetic Approach. New York: Wiley. https://doi.org/10.1002/jps.2600720334