Table of Contents
ToggleSoft ionization Vs Hard ionization
There have been developed different ionizing methods in mass spectrometry, all these can be classified into two major types’ viz. soft ionization and hard ionization. In the hard ionization approach, the molecular ion fragments so extensively that peak due to molecular ion is not detected in most cases. However, in the soft ionization method, there is little to no ion fragmentation, hence ion associated with the intact molecule is usually detected.
Electrospray ionization is a soft ionization technique widely used ionization technique in mass spectrometry. The electrospray ionization technique was first reported by Masamichi Yamashita and John Fenn in 1984.
Electrospray ionization principle
Electrospray ionization has been mostly used soft ionization technique in mass spectrometry for liquid samples throughout the past three decades.
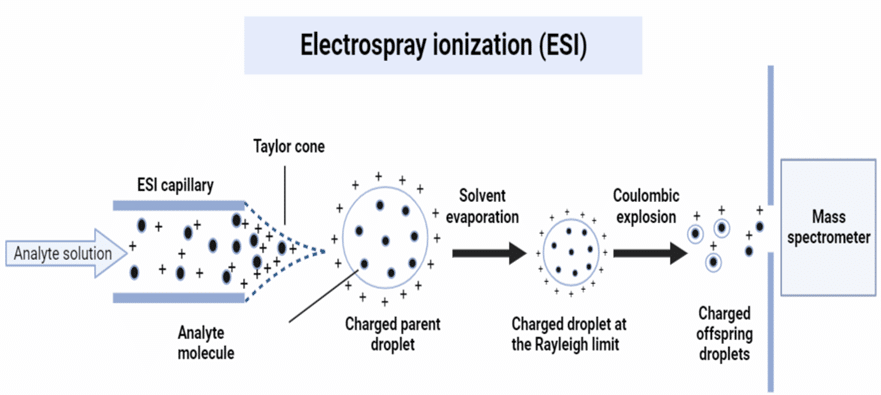
In ESI-MS, electro-spraying or electrospray nebulization is accomplished by applying a high voltage (1-5kV) across the ion-sampling aperture to the ESI capillary. A small steel capillary tube is used to pump the column’s eluent into an open source chamber that is maintained at atmospheric pressure thus forming fine spray-charged droplets. During this process, neutral analyte molecules are charged either in protonation [M+H]+ or cationization by metal, for example; [M+Na]+ in the (+)-ESI ionization method, and deprotonation [M-H]– in the (-)-ESI ionization method. Thus, this method is applicable only for those molecules having either acidic or basic functionalities such that molecules get ionized by deprotonating or protonation.
The formed fine-charged droplets during the electrospray phenomenon undergo desolvation with heated N2 gas flow and cause to shrink in size. With the concomitant reduction in the size of the droplet, the charge density inside the droplet increases leading to a rise in the electrostatic repelling force due to the distribution of like charges. On decreasing the size of the droplet, the electrostatic repulsion inside the droplet and the surface tension of the droplet become exactly equal, such a situation is known as the Rayleigh limit.
A situation is achieved in which the electrostatic repulsion becomes dominant over the droplet’s tension on its surface, which causes the droplet to split and eject multiple tiny charged particles. Such a process is called a columbic burst, which results in the development of the aerosol stream. As the solvent continues to evaporate, free gaseous ions are eventually formed and are driven toward the ion-sampling aperture.
Electrospray ionization mechanism
Even though the actual mechanism of ESI is unknown, Charge Residue Model (CRM) and Ion Evaporation Model (IEM) are two hypotheses that describe the ESI process.
- Charged residue model (CRM)
- Ion Evaporation Model (IEM)
Charged Residue Model(CRM)
According to Charged Residue Model(CRM), a large droplet undergoes solvent evaporation-Columbic fission several times until minute charged droplets of radius 1 nm having only one analyte molecule are formed. Such minute droplets are subjected to additional solvent evaporation until a solvent-free analyte ion is produced, while the droplet’s residual charge is retained.
Ion Evaporation Model(IEM)
In IEM, the liquid-phase analyte ions in contrast escape from the droplets. A charge or many charges are taken from the droplet as they escape. A droplet’s electric field is sufficiently strong to cause solvated charged analyte ions to exit charged droplets once it has undergone enough solvent evaporation-columbic fission phenomenon. Thus, gas phase ion is produced by further desolvation.
Adduct formation in electrospray ionization
The ions detected by mass spectrometry may be quasimolecular ions, which are denoted [M+H]+ when a hydrogen cation is added, [M+Na]+ when a sodium ion is added, or [M-H]– when a hydrogen nucleus is removed. Multiple-charged ions, such as [M+nH]n+, are frequently seen. Large macromolecules may have several charge states, creating a distinctive charge state envelope.
Electrospray ionization diagram
An electrospray or one of its variations serves as the ion source for an ESI-MS apparatus, which also includes an ion optics system for focusing the ions, one or more mass analyzers for separating the ions, a collision cell for fragmenting the ions, and a detector for detecting the ions. To make it easier to analyze structural isomers that could otherwise result in isobaric interference, several instrument vendors additionally offer systems with integrated ion mobility spectrometry (IMS) cells.
1) Sample ionizer
2) Ion source
3) Vacuum system
4) Mass analyzer
5) Detector
Electron impact ionization vs electrospray ionization
The major differences between electron impact ionization and electrospray ionization are listed below.
| Electron impact ionization | Electrospray ionization |
| In electron impact ionization, ionization of the analyte by using a highly energized electron beam occurs. | In electrospray ionization, the ionization of the analyte by using an electrospray nebulizer. |
| The analyte molecule loses one loosely bound electron from the valence shell forming M+. | The analyte molecule either loses a proton [M-H]+ or accepts a proton [M+H]+ during ionization. |
| It is a hard ionization technique that involves the fragmentation of analyte molecules so extensively. | It is a soft ionization technique that does not involve the fragmentation of the analyte molecules. |
| The detection of molecular ion peaks is difficult due to extensive fragmentation. | The detection of molecular ion peaks is possible due to its soft ionization nature. |
Advantages of electrospray ionization
This ionization technique offers the following advantages.
- ESI is a soft ionization technique thus allowing the detection of molecular ion peaks without fragmentation.
- This ionization can achieve high sensitivity and enables the identification and analysis of analytes with low abundance that are found within complex mixtures.
- ESI can be operated in either positive or negative mode thus this feature provides additional flexiblity in mass spectrometric experiments.
- ESI can be used in conjugation with the tandem mass spectrometry technique. Hence, it allows us for the structural elucidation of molecules by fragmenting the ions.
- This technique can be used for a wide range of analytes with heteroatoms(O,N,S,etc)
Disadvantages of electrospray ionization
Although ESI offers many advantages, it has some disadvantages too. Some are given below.
- ESI technique is mainly suitable for the detection of polar molecules. Therefore, it is less effective for non-polar molecules. For the detection of non-polar molecules, GC/MS is preferred over LC/MS.
- Another demerit of ESI is ion suppression. This may lead to the suppression of valuable analyte signals.
- ESI is highly sensitive to contaminants and matrices. This leads to complications in the analysis of spectra.
Application of electrospray ionization
The Electron spray ionization technique is a widely used technique this can be applied in both modes: positive and negative modes. This technique has wide applications in different fields.
- The ESI technique is widely employed in proteomics research to identify, quantify, and characterization of proteins and peptides.
- This ionization technique finds its application in metabolomics experiments to identify small metabolites present in biological samples as well as crude samples.
- It has also an application in lipidomics for the identification and characterization of lipids aiding in the discovery of lipid-related disease.
- One of the rapidly growing applications of ESI is in the biomarker identification of certain diseases.
- Similarly, it has significant utilization in pharmaceutical analysis, food analysis, and environmental analysis.
“Special Thanks To Padam BudThapa“
Electrospray ionization video
References:
- Yamashita, M., & Fenn, J. B. (1984). Electrospray ion source. Another variation on the free-jet theme. The Journal of Physical Chemistry, 88(20), 4451–4459. https://doi.org/10.1021/j150664a002
- Grimm, R. L., & Beauchamp, J. L. (2002). Evaporation and Discharge Dynamics of Highly Charged Droplets of Heptane, Octane, and p-Xylene Generated by Electrospray Ionization. Analytical Chemistry, 74(24), 6291–6297. https://doi.org/10.1021/ac025889b
- Dole, M., Mack, L. L., Hines, R. L., Mobley, R. C., Ferguson, L. D., & Alice, M. B. (2003). Molecular Beams of Macroions. The Journal of Chemical Physics, 49(5), 2240–2249. https://doi.org/10.1063/1.1670391
- Iribarne, J. V., & Thomson, B. A. (2008). On the evaporation of small ions from charged droplets. The Journal of Chemical Physics, 64(6), 2287–2294. https://doi.org/10.1063/1.432536






