Table of Contents
ToggleIf the reaction is carried out at a constant volume, the heat change in the chemical reaction is equal to the difference in internal energy(∆E). Similarly, if the reaction is studied at constant pressure, the heat change in the chemical reaction is equal to the difference in enthalpy changes(∆H). Because ∆E and ∆H are functions of the system’s state, the heat evolved or absorbed in a particular reaction must be independent of how the reaction occurs. As a result, it is dependent solely on the system’s initial and final states, rather than the stages by which the changes occur. Hess’s law of constant heat summation is the name given to this generalization.
State and explain Hess’s law of constant heat summation
State Hess’s law of constant heat summation
Hess’s law of constant heat summation states that if a chemical reaction may be carried out in two or more ways, whether in one step or two or more, the total heat change is the same regardless of the number of steps involved.
In other words, the heat change of an overall reaction is the sum of the heat change of the individual steps involved. This law is based on the first law of thermodynamics.
This law can be understood by studying the following example. Let a chemical substance A can be transformed into Z directly.
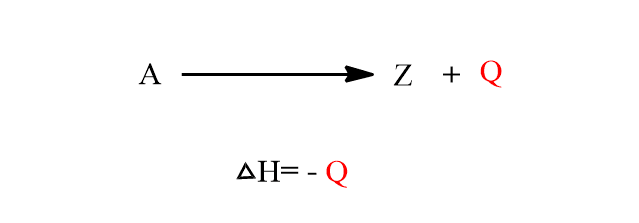
where Q= heat evolved in the direct change of A to Z.
Suppose this transformation of A to Z can be done in following steps:
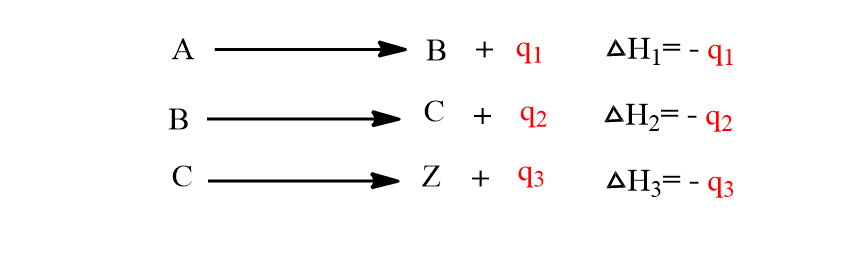
Now, according to this law, Q=q1+q2+q3
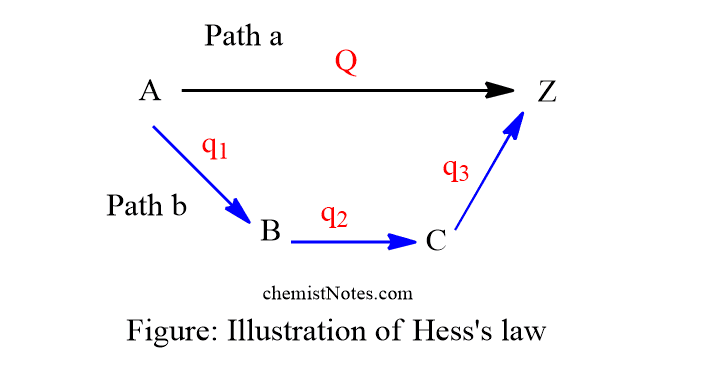
Illustration of Hess’s law
This law can be verified experimentally. If the change in heat energy doesn’t equal the sum of the heat energy of individuals’ steps, this goes against the first law of thermodynamics. Hence, Q=q1+q2+q3
Applications of Hess’s law of constant heat summation
- This law can be used to determine the heat of the formation of a substance that cannot be measured experimentally. For example, the formation of benzene can not be prepared by combining the individual atoms such as carbon and hydrogen. In such a case, the heat of the formation of benzene can not be determined directly. Therefore, Hess’s law is applicable in such cases to determine the heat of formation indirectly.
- The heat of transition of one allotropic form to another can also be determined with the help of Hess’s law.
Hess’s law video
References
- P. Atkins and J. de Paula, Atkins’ Physical Chemistry, (10th Edition), Indian edition,
Oxford University Press, 2014 - Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012






