Table of Contents
ToggleThermodynamics is a branch of science which deals with energy changes and its relationship with work. It is concerned with the transfer of energy from one place to another and from one form to another.
Laws of Thermodynamics
The fundamental principles of thermodynamics are expressed in four laws.
Zeroth Law of Thermodynamics
Zeroth law of thermodynamics states that “If two thermodynamic systems are in thermal equilibrium with some third system, they are also in equilibrium with each other.”
First(1st) Law of Thermodynamics
First law of thermodynamics states that “Energy can neither be created nor destroyed, It can, however, be transferred from one location to another and converted to and from other forms of energy.”
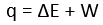
Heat(q) :
If heat flows from the surroundings to the system the energy of the system the energy of the system is raised and is taken to be +ve i.e. +q. If heat flows from the system to the surrounding the energy of the system is lowered and is taken to be negative i.e -q.
Internal energy(ΔE)
If the system absorbs heat its internal energy is increased i.e. +ΔE and if the system loses heat its internal energy decreases i.e. -ΔE.
Work(W)
If work done by the system to the surrounding it is taken to be positive i.e. +W. If the work done on the system by the surroundings is taken to be negative i.e. -W.
Second(2nd) Law of Thermodynamics
Second law of thermodynamics states that “If the physical process is irreversible, the combined entropy of the system and the environment must increase. The final entropy must be greater than the initial entropy for an irreversible process.”
Third(3rd) Law of Thermodynamics
Third law of thermodynamics states that “The entropy of perfectly or purely crystalline solid at absolute zero of temperature is taken as zero.”
Entropy of ice and supercooled liquid (glass) is not zero at absolute zero of temperature, since they are not perfectly crystalline.
FAQs
What is thermodynamics?
Thermodynamics is a branch of science which deals with energy changes and its relationship with work.
What is the first law of thermodynamics?
First law of thermodynamics states that “Energy can neither be created nor destroyed, It can, however, be transferred from one location to another and converted to and from other forms of energy.”
What is the second law of thermodynamics?
Second law of thermodynamics states that “If the physical process is irreversible, the combined entropy of the system and the environment must increase. The final entropy must be greater than the initial entropy for an irreversible process.”
What is the third law of thermodynamics?
Third law of thermodynamics states that “The entropy of perfectly or purely crystalline solid at absolute zero of temperature is taken as zero.”
What is q in thermodynamics?
In thermodynamics, q is represented for heat.






