Primary and secondary processes are two types of processes present in photochemical reactions. These two processes are discussed simply and in short below:
Primary process in photochemistry
The primary process of the reaction is the light-absorbing process which follows the law of photochemical equivalence. Actually, the primary process is the photoexcitation or photoactivation of reacting molecules by the absorption of light.
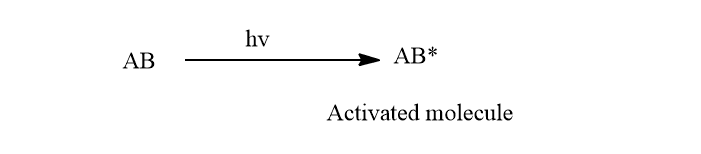
As previously stated, electronic excitation is caused by a photon absorbed by a molecule. The frank codon principle governs the electronic transition. According to this theory, the time necessary for electronic excitation is so short that the internuclear distance remains constant throughout the process.
Based on the interaction between the lower and upper electronic levels, four different main processes are possible. These are discussed briefly in order as:
- Type I: In this situation, the molecule’s vibrational energy in the upper electronic state surpasses the maximum value, causing molecules to dissociate in the first oscillation. The electronic spectrum is made up of discrete vibration-rotation bands separated by a continuous absorption region.
- Type II: The molecule is simply stimulated to the highest electronic state in this situation. The electronic spectrum is made up of a number of bands connected by a continuous region.
- Type III: The molecule gets dissociated once more since the highest level indicates an unstable state. The electronic spectrum is continuous all the way through.
- Type IV: In this situation, the stable and unstable higher levels overlap, resulting in a transition from the lower levels to the stable top level. During vibration, the molecule switches to the unstable state at the point where two levels cross, and the molecules subsequently dissociate. Predissociation is the term used to describe this behavior. A banded region exists in the electronic spectrum.
Secondary process in photochemical reaction
The primary process’s byproducts may be involved in a subsequent thermal reaction. These are referred to as secondary processes. It means, excited molecules, free radicals produced in the primary process of absorption of radiation, initiates a series of reaction called the secondary process.
The secondary process may involve only one or more than one steps. As discussed before, the secondary processes represent the chain reaction. Due to such a secondary process involved in a photochemical reaction, the reaction generally has a quantum yield value greater than one.
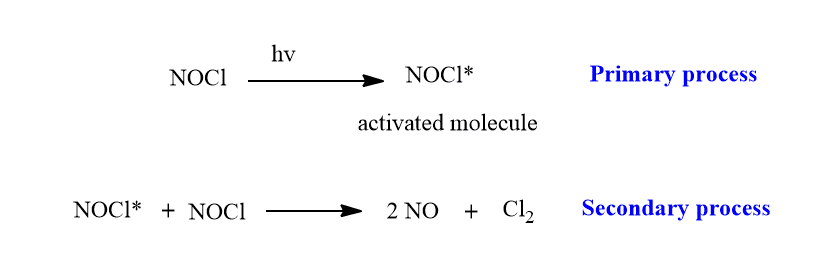
For example, the photochemical decomposition of NOCl involves the following processes.