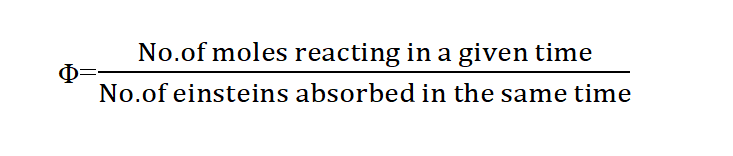Table of Contents
ToggleQuantum yield, also known as quantum efficiency is an important parameter that measures the efficiency of a photochemical reaction.
Quantum yield
Quantum yield is defined as the number of molecules reacting per quantum of light absorbed. It is denoted by Φ.
In another word, Quantum efficiency is defined as the number of moles of the light-absorbing substance that react chemically for each einstein of absorbed radiation.
Mathematically, Quantum yield can be expressed as:
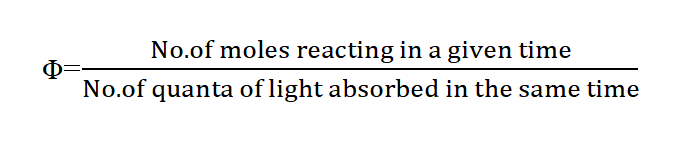
If the law of photochemical equivalence is true, Φ should have a value of 1. Since the value of Φ may vary from zero to 106, It was realized that the law of photochemical equivalence is applicable to the only primary process.
There are two types of photochemical reactions on the basis of quantum yield. One is a high quantum yield reaction and another is a low quantum yield reaction.
High quantum yield
A reaction is said to have a high quantum efficiency if the value of Φ is greater than 1 for that reaction. Let’s see some examples of such reactions.
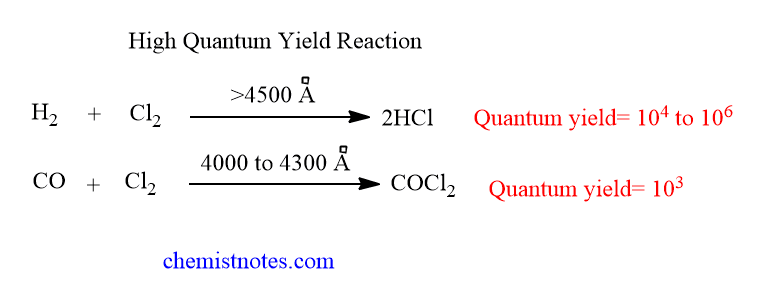
Reasons of high quantum yield
There are some reasons which may be responsible for the high quantum efficiency.
- The primary process of absorption of radiation produce excited atoms,molecules or free radicals which initiates a series of chain reaction called secondary processes. Thus, by absorbing only one quantum of radiation, several reactant molecules undergo chemical reaction. Hence Φ will be greater than unity.
- Formation of an intermediate product acts as a catalyst and readily propagate the reaction.
- The secondary reaction may be exothermic which activates other secondary process as a result more reactant molecules undergo chemical change without absorption of radiation
Low quantum yield
A reaction is said to have a low quantum efficiency if the value of Φ is less than 1 for that reaction. Let’s see some examples of such reactions.
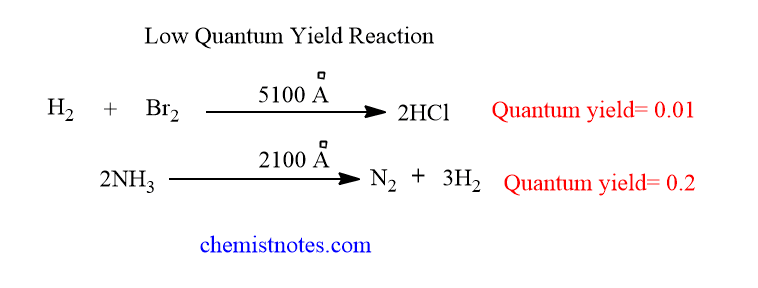
Reasons for low quantum yield
Some photochemical reactions are reported to have very low quantum efficiency and the reason for such phenomenon are given below:
- If excited molecules formed in primary process are such that they cant react due to their deactivation by collisions or by internal arrangement, the quantum yield will be extremely low.
- Collision of excited molecules with non-excited molecules may cause to loss their energy. This is another cause of low quantum yield.
- The excited molecules produced in the primary process may recombine to form the reactant so as to give low quantum yield.
- If a reacting molecules Is initially present at such a low energy level, so that it does not aquire an optimum energy level to take part in photochemical process by photoexcitation.
- Some of the photochemically excited molecules in primary process donot undergo secondary reaction. Thus, there is some time interval between primary and secondary process. And they lose some energy. This will give low quantum yield.
How to measure quantum efficiency ?
In order to calculate the quantum efficiency experimentally, we must measure the number of moles of the reacting substance that undergoes a chemical change in a given time and the number of einsteins of radiation absorbed by the light-absorbing substance during that time.
The experimental setup for the determination of quantum efficiency is shown as:
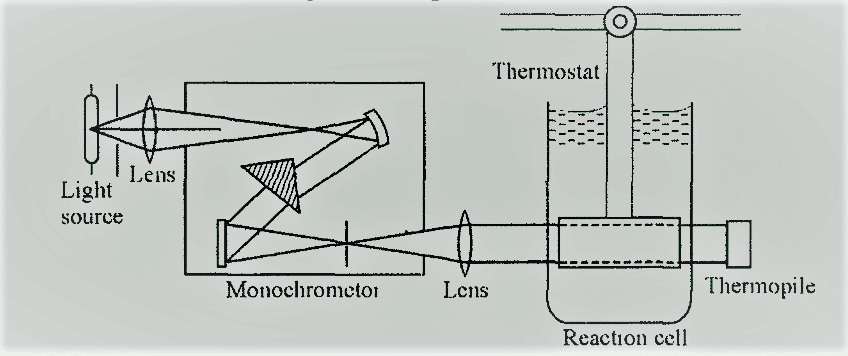
First of all, the empty cell or the cell filled with solvent is placed in the path of the light beam and the intensity of light is measured. Then, the reaction cell is filled with the reaction mixture. The light is passed through the reaction cell for a certain period of time. After then, the intensity of light is measured after the completion of the reaction.
The difference between these two data will give the total energy absorbed by the reaction system in the given time period. The intensity of the radiation absorbed is given by the total energy absorbed divided by the volume of the reaction mixture if the time is one second. Then compute the number of moles that reacted in a given time period. The following formula is used to compute the value of Φ.