Table of Contents
ToggleWhen certain substances are exposed to light, they emit light for a period of time after the light source is cut off. This phenomenon is known as phosphorescence and such substances are called phosphorescent substances. Generally, solid substances such as zinc sulphide, and sulphide of alkaline earth metals exhibit this phenomenon as the molecules have the least freedom of motion so that excited electrons keep on jumping back slowly for quite some time.
Phosphorescence is considered a slow fluorescence since it occurs when fluorescence sustains for a long time.
Principle of Phosphorescence
When a molecule in the ground state (singlet) absorbs UV or visible radiations of the proper frequency, one electron passes into the vacant orbital present at a higher energy level.
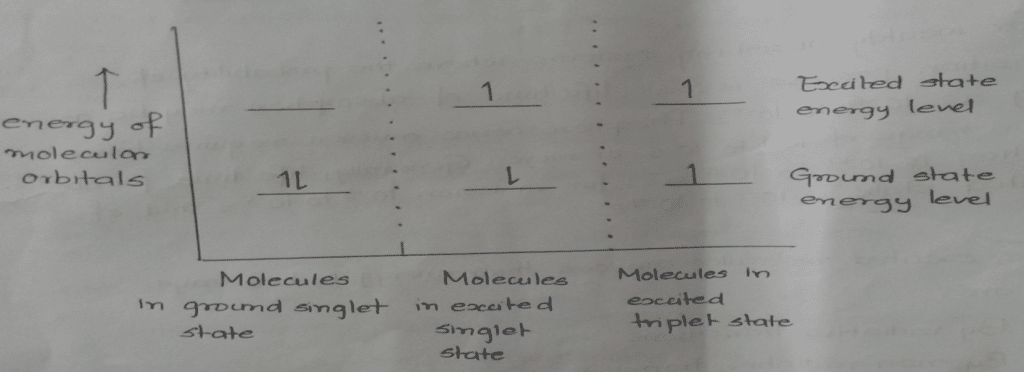
If the spin of the promoted electron does not undergo any change and the net spin is still zero. This is called the excited singlet state, while if the promoted electron has a spin parallel to its original partner, then it is called the excited triplet state.
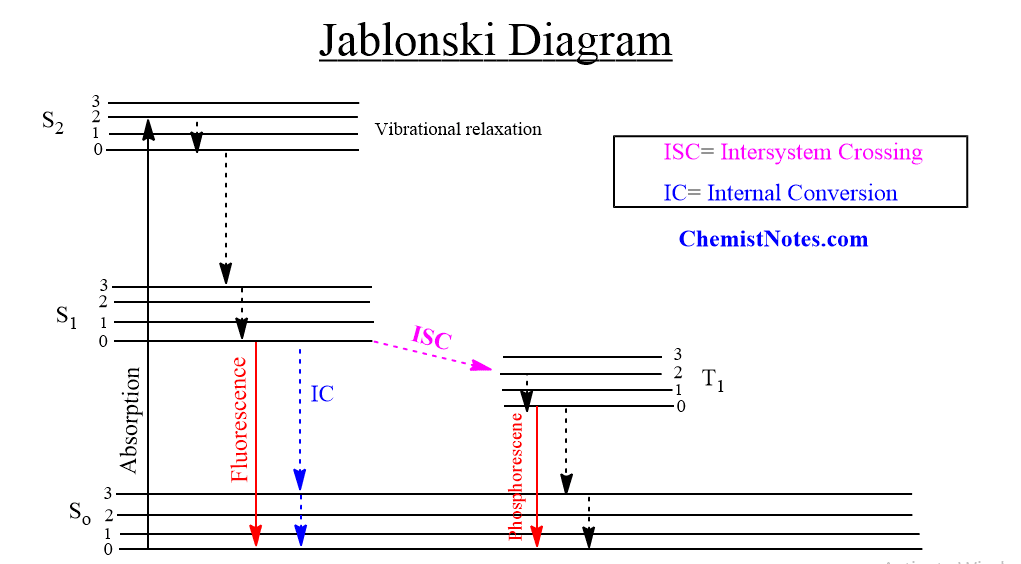
When incident light strikes a phosphorescent substance, the molecules absorb energy hv and are excited from the singlet ground state (So) to the singlet excited state (S1). Through intersystem crossing, a singlet excited electron transits to the triplet state by vibrational relaxation. [Note: the energy of the excited triplet is lower than the energy of the corresponding singlet state]. Direct transition from the ground singlet electronic state to the triplet electronic state is impossible because this leads to a change in multiplicity and thus has a low probability of occurring which is a forbidden transition.
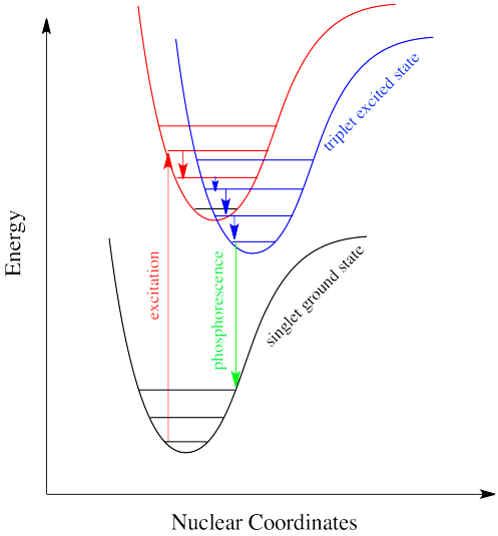
Because the transition from triplet (T1) to singlet (ground state) is also forbidden as the electron spin in the ground state is parallel to the spin in the triplet excited state, the excited molecules stay in the triplet state (T1) for a longer time. This time could be about a few seconds, minutes, or even hours. The electron, then eventually come back down, accompanied by the emission of a photon. This phenomenon is called “phosphorescence“, and can be illustrated in the Jablonski diagram as:
With respect to emission, the average lifetime of the excited triplet state ranges from 10-4 S to 10 S or more. As a result, emissions from such a transition may occur even after irradiation has stopped.
Application of phosphorescence
- In the determination of organic compounds like hydrocarbon, pesticides, and petroleum products.
- For the manufacture of luminous paints that are used in dials, electric switches, and so on.
- Useful in toys, make-ups, safety signs, radar screens, and so on.
- Determination of aspirin (acetylsalicylic acid) in blood serum.
- Highly applicable in the pharmaceutical industry and forensic analysis.
- Cocaine and atropine in urine have been determined by the phosphorescence technique in combination with extraction procedures.
Difference between fluorescence and phosphorescence
| Fluorescence | Phosphorescence |
| Fluorescence is a radiative relaxation process in which light is emitted as a result of the transition from S1 (excited singlet state) to So (ground singlet state) without change in spin multiplicity. | Phosphorescence is a radiative transition process in which light is emitted from T1 (excited triplet state) to So (ground singlet state) with a change in spin multiplicity. |
| When certain substances are exposed to light, they stop emitting light instantly after the light source is cut off. | They emit light for a period of time even after the light source is cut off. |
| It is shown by gases, liquids, and solids. | Only solids exhibit this phenomenon. |
| Less selective. | More selective. |
| It is experimentally preferred because it is less complicated. | Less preferred due to more complications. |
| It doesn’t involve a change in spin multiplicity. | It involves a change in spin multiplicity. |
| Excited-state has a short lifetime (10-7 – 10-9 s). | Excited-state has a long lifetime (10-4 – 101s). |
Phosphorescence Video
FAQs/MCQs
What is phosphorescence?
When certain substances are exposed to light, they emit light for a period of time after the light source is cut off. This phenomenon is known as phosphorescence
phosphorescence examples
Zinc sulphide, and calcium sulphide, along with other suplhides of alkaline earth metals are examples of phosphorescence.
what is fluorescence and phosphorescence?
Fluorescence is a radiative relaxation process in which light is emitted as a result of the transition from S1 (excited singlet state) to So (ground singlet state) without change in spin multiplicity.
Phosphorescence is a radiative transition process in which light is emitted from T1 (excited triplet state) to So (ground singlet state) with a change in spin multiplicity.
References
- Charles DePuy and Orville L. Chapman, Molecular Reaction and Photochemistry,
Prentice-Hall, 1972. - Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- Atkins Physical chemistry 8th. Peter Atkins. Julio De Paula. Ch. 13 p.567-569






