Table of Contents
ToggleLe-chaterlier’s principle is a generalization made by a French chemist Le-chatelier to explain the qualitative effect of changes in concentration, temperature, or pressure on the state of the system in equilibrium.
State Le chatelier’s principle
According to Le-chatelier’s principle, If a system in equilibrium is subjected to a change of concentration, pressure, or temperature, the equilibrium shifts in the direction that tends to neutralize the effect of the change.
The main statements of this principle are as follows:
- The increase in temperature favors the reaction which takes place with the absorption of heat. It means an increase in temperature will shifts the equilibrium in the forward direction for an endothermic reaction. But the equilibrium will shift in the backward direction if the reaction is an exothermic reaction. Thus, the effect of temperature in equilibrium depends on the nature of the reaction whether the reaction is either endothermic or exothermic.
- The increase of pressure shifts the system in the direction in which there is decreased in volume. and vice versa.
- Increase in concentration of reactant shifts the equilibrium in forward direction but an increase in the concentration of product shifts the equilibrium in the backward direction and vice-versa.
What will be the effect of the addition of inert gas to the equilibrium system? Well, on mixing inert gas the volume of the reaction vessel does not change. On the other hand, the addition of the inert gas increases the total pressure but the partial pressures of the reactants and products are not changed. Therefore, the addition of inert gas has no effect on the equilibrium system.
Le chatelier’s principle application
Le chatelier’s principle is not only applicable to chemical equilibrium but is also applicable to physical equilibrium. Let’s discuss with suitable examples.
Application of Le-chatelier’s principle to physical equilibrium
As stated above, this principle can be applied to physical processes which are in equilibrium. Let’s explain how this law is applied to study the effect of temperature, and pressure in the Melting of ice.
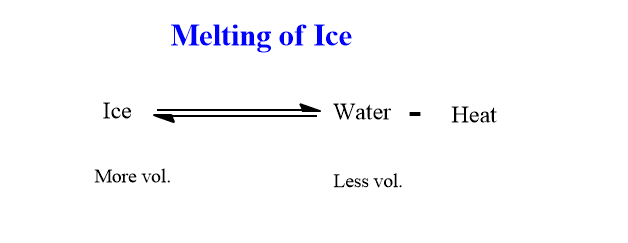
When ice absorbs heat, it melts with a decrease in volume. It means, the process is endothermic and there is a decrease in the volume of water when the ice melts.
This is an endothermic process, therefore increase in temperature shifts equilibrium in the forward direction i.e melting of ice. Hence, by applying heat energy the equilibrium can be shifted towards the melting of ice. i.e more ice melts into water.
Similarly, we know the volume of water is always less than that of ice under the same conditions. Hence, with increasing pressure, the system will shift toward forward direction.
Application of Le-chatelier’s principle to chemical equilibrium
Let us explain by taking an example of ammonia synthesis by Haber’s process.
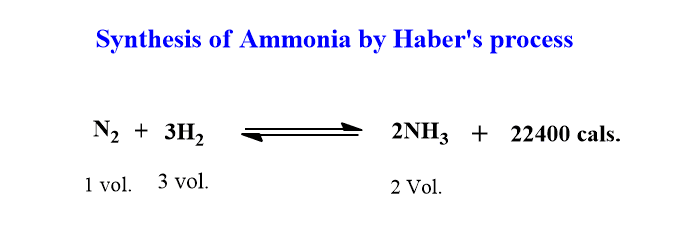
Effect of temperature: The reaction is exothermic and an increase in temperature will shift the equilibrium in the background direction. It means it will result in the decomposition of ammonia. Therefore, a decrease in temperature favors the formation of ammonia. Hence, for high yield, the reaction should not be high and maintained at low temperatures.
Effect of pressure: There is 1 volume of nitrogen which reacts with 3 volumes of hydrogen to give 2 volumes of ammonia. So, there is 4 volume of reactant and 2 volume of products. Therefore, an increase in pressure will favor the reaction in the forward direction so that more ammonia is formed.
Effect of concentration: When the concentration of N2 or H2 is increased, the equilibrium shifts in that direction where there is a decrease in their concentration. i.e in the forward direction which means the equilibrium will shift towards forwards forming more ammonia.
Therefore, the formation of ammonia is favored by high pressure, low temperature, and excess of N2 or H2.






