Table of Contents
ToggleThomas Graham formulated a law in 1829 to describe the relationship between the rate of diffusion and the densities of gases. This law is known as Graham’s law of diffusion. The process of spontaneous mixing of two or more gases by the random motion of the molecules is called diffusion.
Graham’s law of diffusion states that “Under similar conditions of temperature and pressure, the rate of diffusion of gases are inversely proportional to the square root of their densities“.
Graham’s law of diffusion
If r1 and r2 are the rates of diffusion of two gases, and d1 and d2 are their respective densities, then according to Graham’s law,
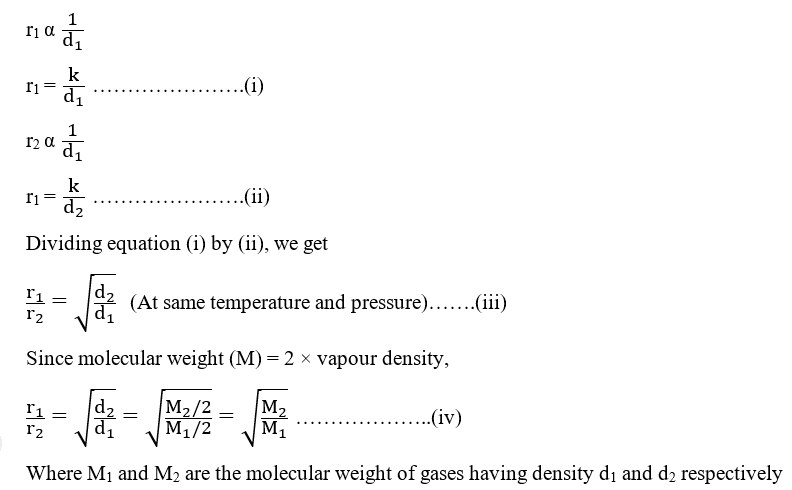
Thus, Graham’s law also stated as, “Under similar conditions of temperature and pressure, the rate of diffusion of gases are inversely proportional to the square root of molecular weights“.
As the rate of diffusion of a gas is equal to the volume of the gas diffused per unit of time
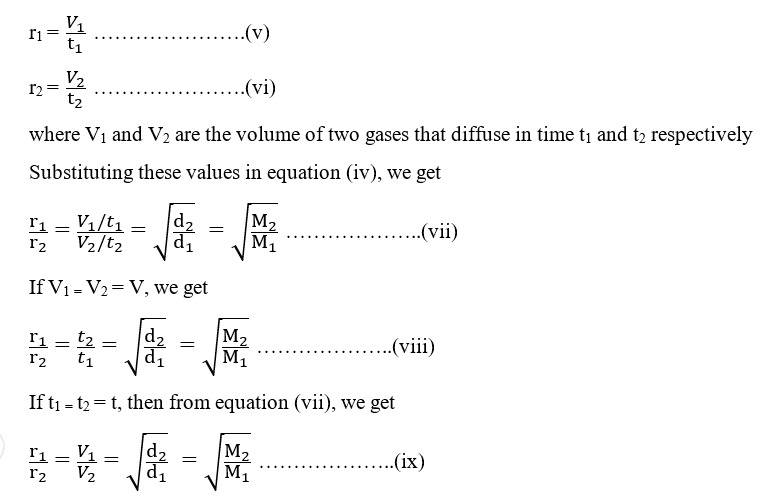
Thus, under similar conditions of temperature and pressure, the time taken for the diffusion of an equal volume of two gases is directly proportional to the square root of their densities or molecular weights. From equation (ix), it can be also concluded that volumes of two gases that diffuse at the same time under the similar condition of temperature and pressure are inversely proportional to the square root of their densities or molecular weights.
Graham’s Law of Effusion
Effusion occurs when a gas is allowed to escape from its container through a pinhole or small aperture into a low-pressure vacuum zone. This process is known as effusion. Graham’s law, when applied to the effusion of a gas, is called Graham’s Law of Effusion and is found that the rate of effusion of gases depends on the molecular weight under similar conditions of temperature and pressure.
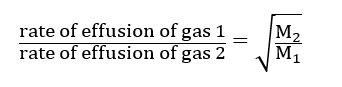
Application of Graham’s law of diffusion
- For the partial separation of the components in a gas mixture.
- Determination of density or molecular weight of gases.
- Separation of isotopes of certain elements like chlorine, bromine, oxygen, and so on.
Graham’s Law video
References
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.
- R.H. Petrucci, W.S. Harwood, and F.G. Herring, General Chemistry (8th ed., Prentice-Hall 2002) pp. 206–08






