Table of Contents
ToggleHittorf method for determination of transport number is one of the commonly used methods, which is based on Hittorf’s rule. Actually, hittorf’s rule state that loss of concentration around an electrode is proportional to the speed of the ions moving away from that electrode. The transport number of an ion is determined experimentally from the changes in the concentration of ions around the electrode.
Hittorf method for determination of transport number
The required Hittorf’s apparatus consists of two vertical glass tubes joined together through U-tube in the middle. There are stopcocks at the bottom of all three tubes. But, the stopcocks are also present at the tops of the two limbs of the U-tube as shown below.
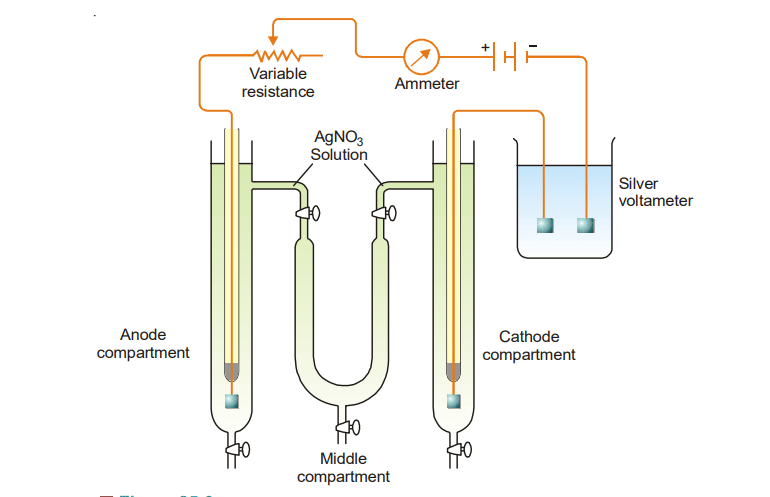
These stopcocks are used to communicate between the solution in the cathode and anode limbs. The whole apparatus is filled with a solution of silver nitrate and a very small constant current is passed for two or three hours. Current is passed only for a short time to avoid a large change in concentration.
After passing the current for 2-3 hours, the stopcocks at top of the U-tube are closed. Then the whole liquid of the anode compartment is carefully drained into a weighted flask and its weight determined. The silver content of the solution is determined by titrating against standard solutions of potassium thiocyanate. The weight of silver deposited in the silver coulometer is also noted.
Note: If a copper coulometer is used in the place of a silver coulometer, the weight of silver equivalent to the copper deposited is calculated by multiplying it by 108/31.5
Precautions:
- There should be no more change in the concentration of the solution in the solution in the U-tube if the experiment has been successfully performed.
- If the experiment has been performed by using a silver electrode, nitrate ions attack the silver anode. Consequently, there is an increase in the concentration of Ag+ ions rather than a decrease. To avoid the attack of anions on the electrode, an experiment can be carried out by using platinum electrodes.
Calculation of transport number by hittorf’s method
There are two different cases. One case is for an unattackable electrode and another for an attackable electrode.
Case 1: When electrodes are unattackable
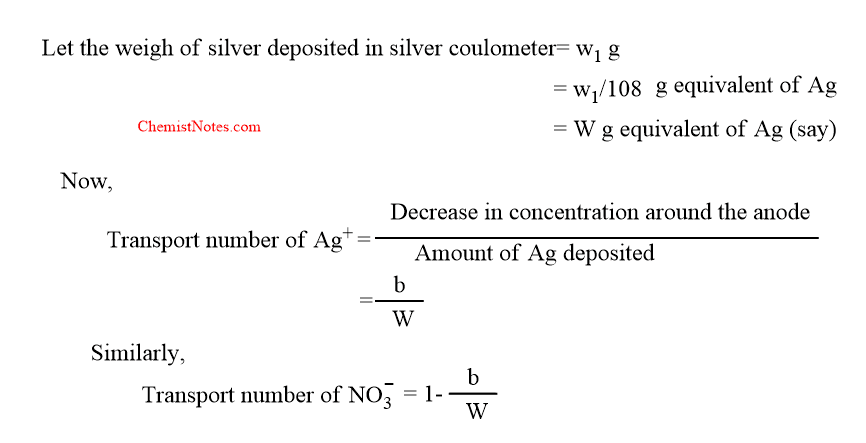
After passing the electric current, let the weight of the anodic solution be taken out= x gram
weight of silver nitrate present in it determined by titration = y gram
so, the weight of water = (x-y) gram.
Let the weight of AgNO3 present in (x-y) g of water before passing electric current = z g
Therefore, decrease in concentration = (z-y) g of AgNO3= (z-y)/170 g equivalent of AgNO3=(z-y)/170 g equivalent of Ag= say b
Case 2: When electrodes are attackable
Increase in concentration of anodic solution= (y-z) g of AgNO3
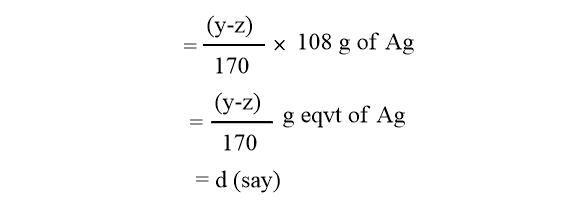
The rise in Ag+ ion concentration would have been equal to W if no Ag+ ions had migrated from the anode.
Now, decrease in concentration due to the migration of Ag+ ion= W-d
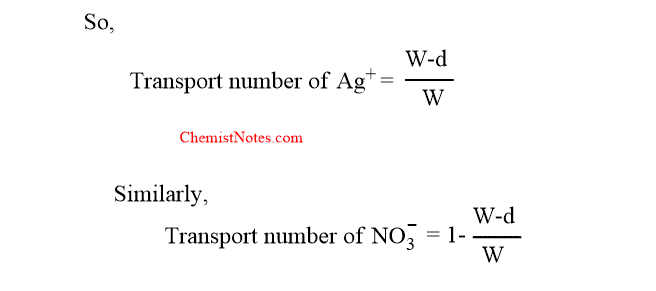
Hittorf’s method youtube video
References
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.
- J. N. Gurtu and A. Gurtu, Advanced Physical Chemistry Experiments, (6th Edition), Pragati Prakashan, Meerut, India, 2014.






