Table of Contents
ToggleMichael Faraday (1834) proposed a quantitative relationship between the quantity of electricity passed through electrolytes and the amount of the substance liberated at an electrode. This law of electrolysis is called Faraday’s law of electrolysis. The phenomenon of decomposition of an electrolyte in the molten or aqueous state by passing an electric current through them is called electrolysis. Faraday formulated two laws:
- Faraday’s First law of electrolysis
- Faraday’s second law of electrolysis
Faraday’s first law of electrolysis
State Faraday’s first law of electrolysis
Faraday’s first law of electrolysis states that “the mass of the substance deposited or liberated at an electrode is directly proportional to the quantity of charge passed through it”.
If ‘m’ is the mass in grams of a substance deposited on an electrode by passing electricity ‘Q’ coulombs, then
m ∝ Q
m ∝ It [Since, Q = It where I is the current in amperes and t is the time of current flow in second].
been supplied.
m = ZIt
where Z is the called Electrochemical equivalent of the substance or an electrolyte.
If I = 1 ampere and t = 1 second, then
m = Z
The amount of a substance deposited by the 1-ampere current for 1 second is known as the electrochemical equivalent.
Application of Faraday’s first law of electrolysis
- The amount of different deposited substances can be calculated by passing a known amount of electricity through their solutions.
- Used to know the value of electrochemical equivalents of different substances.
Faraday’s second law of electrolysis
State Faraday’s second law of electrolysis
Faraday’s second law of electrolysis states that “when the same amount of charge is passed through electrolytic solutions for the same amount of time, the mass of the different substances deposited at respective electrodes is directly proportional to their chemical equivalents.”
i.e. m ∝ E, where m is the mass of the substance discharged and E is the equivalent weight
m = E × constant
m/E = constant
Verification of Faraday’s Second Law of Electrolysis
Faraday’s second law of electrolysis is verified by passing the same amount of current through a series of electrolytic cells having different electrolytes for the same amount of time.
Let us consider, there voltameters (fitted with platinum, copper, and silver electrodes) containing solutions of dil. H2SO4, CuSO4, and AgNO3 as shown in the figure below. Now, the same quantity of charge is allowed to pass through it. Hydrogen, copper, and silver deposited in corresponding voltameters at the cathode are in the ratio of their equivalent weights.
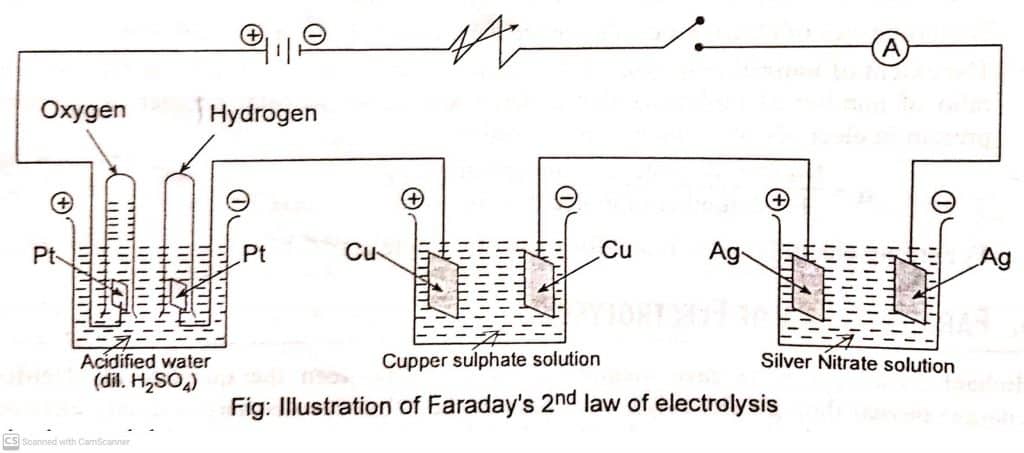

From the above relation, it is clear that the same amount of charge is required to deposit one gram equivalent of substance, and hence verifies Faraday’s second law of electrolysis.
Application of Faraday’s second law of electrolysis
Faraday’s second law of electrolysis is used to calculate equivalent weights of metals, Avogadro’s number, and the unit of electric charge.
Faraday’s law of electrolysis video
FAQs/MCQs
Faraday’s law of electrolysis are related to the
Faraday’s law of electrolysis is related to the equivalent weight of electrolytes since number of Faraday’s passed is equal to the gram equivalent of electrolytes discharged.
Faraday’s second law of electrolysis formula
Faraday’s second law of electrolysis is based on m/E = constant i.e. m ∝ E, where m is the mass of substance deposited and E is the equivalent weight.
State and explain faraday’s law of electrolysis
Faraday’s first law of electrolysis states that “the mass of the substance deposited or liberated at an electrode is directly proportional to the quantity of charge passed through it”.
According to Faraday’s second law, ” when the same amount of charge is passed through electrolytic solutions for the same amount of time, the mass of the different substances deposited at respective electrodes is directly proportional to their chemical equivalents.”
Faraday’s first law of electrolysis formula
Faraday’s first law of electrolysis is based on m ∝ Q, where m is the mass of the substance and Q is the quantity of charge passed.
References
- Faraday, Michael (1834). “On Electrical Decomposition”. Philosophical Transactions of the Royal Society. 124: 77–122. doi:10.1098/rstl.1834.0008.
- Atkins Physical chemistry 8th. Peter Atkins. Julio De Paula. Ch. 13 p.567-569
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.






