Table of Contents
ToggleHeterogeneous catalysis and homogenous catalysis are two categories of catalysis on the basis of the phase of reactant and catalyst. Before explanation of heterogenous catalysis, we must know what is a catalyst? A catalyst is a substance that may be ions, salt, metal, or inorganic compound, whatever that influences the rate of a chemical reaction, but itself remains unchanged chemically at the end of the chemical reaction, the phenomenon is known as catalysis.
A catalyst may be further classified as Positive, Negative, and Autocatalyst on the basis of its activity. Some catalyst increases the rate of reaction, known as a positive catalyst, and some decrease the rate of reaction called a negative catalyst. For example: In the decomposition of potassium chlorate, Mno2 acts as a positive catalyst while in the decomposition of hydrogen peroxide, acetanilide retards the rate hence acts as a negative catalyst.
There is another case, in which one of the products of a reaction acts as a catalyst for that reaction then such a product is called autocatalyst, and the phenomenon is termed autocatalysis. One example of such a type of catalysis is the hydrolysis of ester, in which acid formed during hydrolysis catalyzes the reaction.
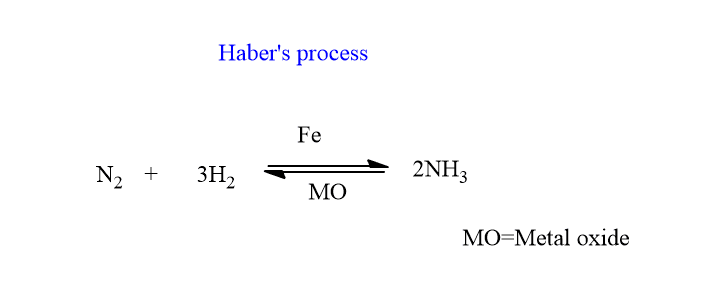
Sometimes we use additional substances with catalysts to carry out certain reactions, have you noticed? Those substances either enhance the activity of the catalyst or retards the function of the catalyst. Thus, such substances which are not a catalyst but enhance the activity of a catalyst in a chemical reaction are called Promoters or activators. One example of such reaction is Haber’s process in which, the Metal oxide is used along with the Iron catalyst.
But what if that substance retards the activity of the catalyst? Such substances are called inhibitors or catalytic poisons. The carbon monoxide acts as a catalytic poison in the synthesis of ammonia by Haber’s process.
Heterogeneous catalysis
The catalysis in which reactant and catalyst are in different phases is called heterogeneous catalysis. A reaction in which reactant may be in gas or liquid phase and catalyst may be in solid-state, Such catalysis termed as heterogeneous catalysis.
In Haber’s process of manufacture of ammonia, Nitrogen and Hydrogen are in the gaseous phase and Iron is present in solid-state, which acts as a catalyst.
Adsorption theory of catalyst
Heterogeneous catalysis generally proceeds via the adsorption of the reactant on the surface of the catalyst. Therefore, this theory is known as the adsorption theory.
According to the adsorption theory of heterogeneous catalysis, first of all, the reactant molecules come in contact with the catalyst surface by diffusion followed by bonding with active centers of the catalyst surface. Due to adsorption, the concentration of reactant molecules increases on the surface, so that they undergo interaction with each other to form an activated complex. The activated complex decomposes to give product and regenerates the catalyst.
To explain this theory, let us take the example of hydrogenation of ethane in presence of Nickel.
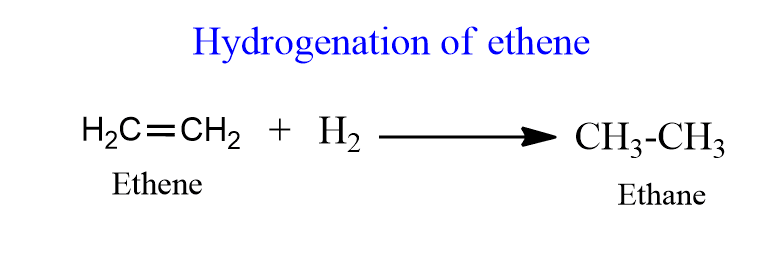
Step 1: Hydrogen molecules get adsorbed on the surface of nickel by their free valence bonds.
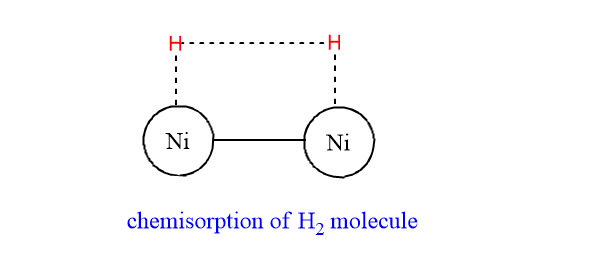
Step 2: H-H bonds are broken and H-atoms are separated.
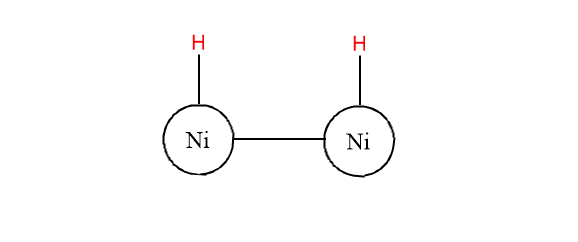
Step 3: Ethene molecules interact with chemically adsorbed H-atom to form an unstable activated complex.
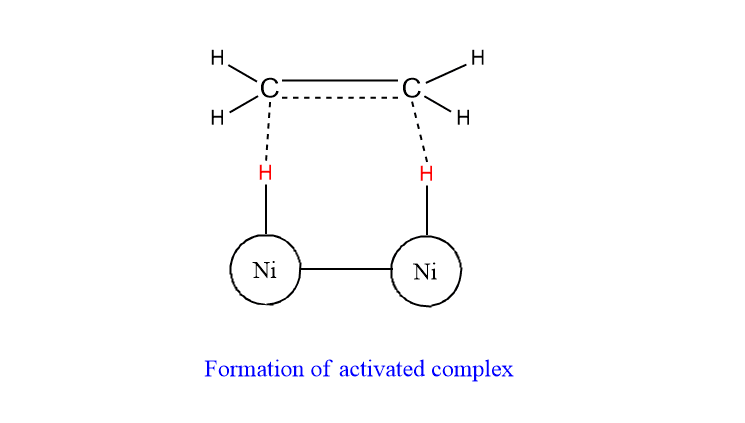
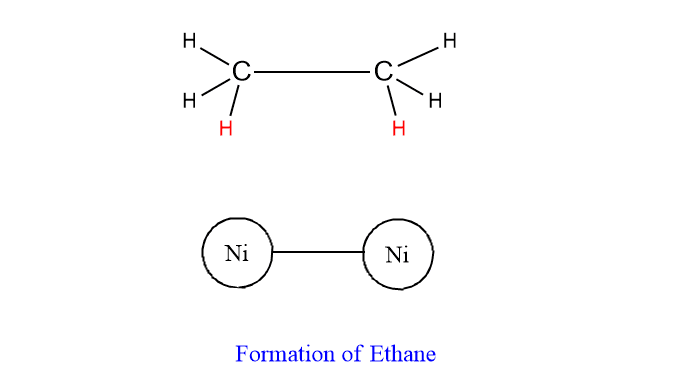
Step 4: The activated complex decomposes to give the product.
Criteria of catalysis
The criteria or characteristics of catalysis/catalyst are listed below:
- A small amount of catalyst is enough to catalyse the rate of reaction.
- The catalyst remains unchanged in amount and composition at the end of a chemical reaction.
- A catalyst does not initiate a chemical reaction.
- A catalyst doest not affect equilibrium condition.
- The action of catalyst is universal and specific.
- The activity of catalyst is enhanced by the presence of a substance called promoter.
- A catalyst has an optimum temperature at which the action of the catalyst is maximum.
- Impurities present in small amount on catalyst destroy the catalytic activity called inhibitors or catalytic poison.
What is a catalyst?
A catalyst is a substance that may be ions, salt, metal, or inorganic compound, whatever that influences the rate of a chemical reaction, but itself remains unchanged chemically at the end of the chemical reaction.
What is a positive catalyst?
A positive catalyst is a type of catalyst that enhances the rate of reaction.
What is a negative catalyst?
A negative catalyst is a type of catalyst that decreases the rate of reaction.
What do you mean by promoter?
substances that are not a catalyst but enhance the activity of a catalyst in a chemical reaction are called Promoters or activators
What is catalytic poison?
A substance that inhibits the activity of the catalyst is called catalytic poison.
Define homogeneous catalysis
If the catalyst and reactants of a reaction are in the same phase, then such catalysis is called homogeneous catalysis.







One Response
Good