Table of Contents
ToggleEquivalent conductance is the conductance of an electrolyte measured after dissolving one gram equivalent in V cc of water. Its definition, units, expression, and its variation with dilution have been discussed below:
Equivalent conductance definition
Equivalent conductance of electrolyte is defined as the conductance of all ions produced by dissociation of one gram equivalent of an electrolyte dissolved in a V cc of the solution when the distance between two electrodes is 1cm and the area of the electrodes is so large that whole solution is contained between them.
Equivalent conductance is denoted by Λ or λ and given by
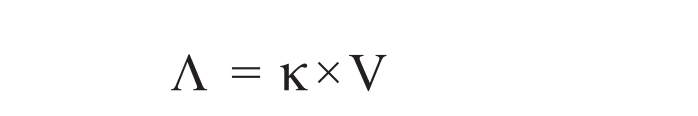
Where, κ= specific conductance, it is also denoted by Ls. Don’t get confused.
V= Volume in cm3 containing 1 gram equivalent of an electrolyte.
If N be the concentration of the electrolytic solution in gram equivalent per liter, then the volume containing 1 gram equivalent of electrolyte becomes (1000/N). So, the above equation can be expressed as,
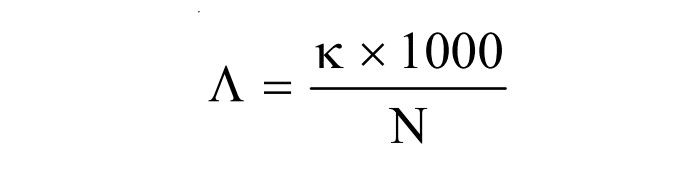
Equivalent conductance at infinite dilution
Equivalent conductance at infinite dilution is the sum of equivalent ionic conductance of ions composing the given electrolytes. This law is known as Kohlrausch’s law of independent migration of ions.
At infinite dilution, electrolytes are almost completely ionized and these ions are far from each other. In such cases, interionic interactions are almost absent, and ionic mobility is high. Therefore, the value of equivalent conductivity is maximum at infinite dilution.
Equivalent conductance at infinite dilution is denoted by Λo and given as:
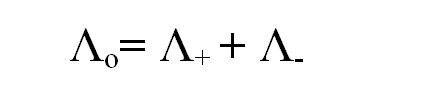
unit of equivalent conductance
The unit of equivalent conductivity can be derived as:
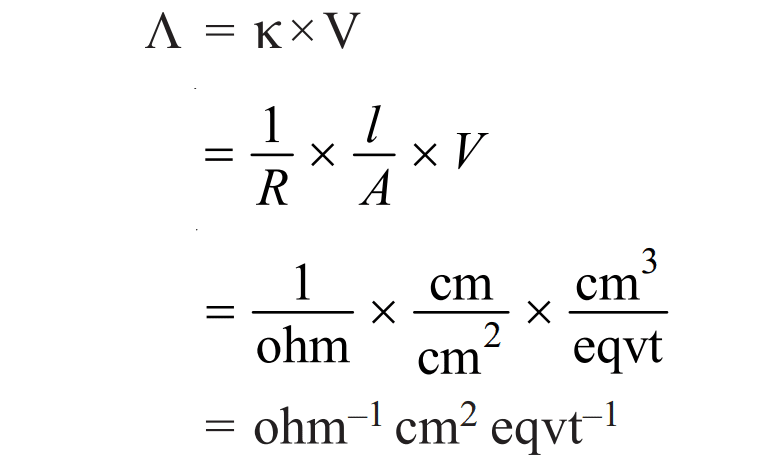
Therefore, the unit of equivalent conductance is ohm-1 cm2 eqvt-1 or ohm-1 cm2 eq-1or mho cm2 eq-1 or S cm2 eq-1. Moreover, the SI unit of equivalent conductance is S m2 eq-1.
Equivalent conductance formula
The formula of equivalent conductance is given as:
Λ= κ × (1000/N).
This is the relation between specific conductance and equivalent conductance.
Effect of dilution on equivalent conductance
Equivalent conductance increases with dilution. An increase in equivalent conductivity is due to an increase in the mobility of ions in a very dilute solution. Similarly, on dilution the number of ions increases. Since equivalent conductivity depends on the number of ions and ionic mobility, both increase on dilution hence equivalent conductivity also increases. This can be represented in the graph as well for strong and weak electrolytes.






