Table of Contents
ToggleSpecific conductance, equivalent conductance, and molar conductance are the terms mostly used in the case of electrolytic solutions. All of these represent the ability of an electrolytic solution to conduct the electricity.
Define specific conductance
Specific conductance is reciprocal of specific resistance. It is denoted by the symbol κ (kappa) or Ls, which is given as:
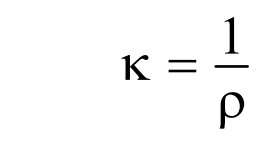
where ρ is specific resistance whose value depends on the nature of the conductor.
We know from Ohm’s law,
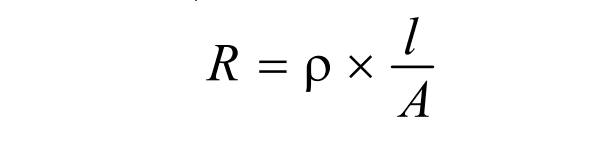
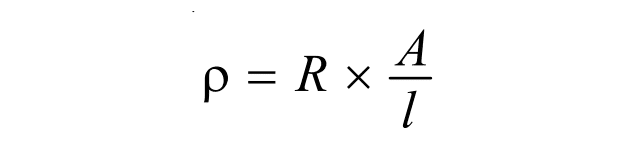
Now, using the value of ρ, the expression of specific conductance becomes,
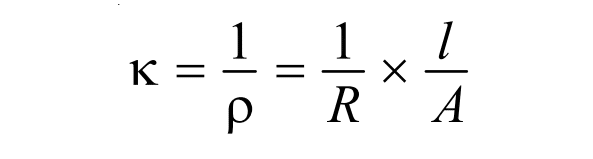
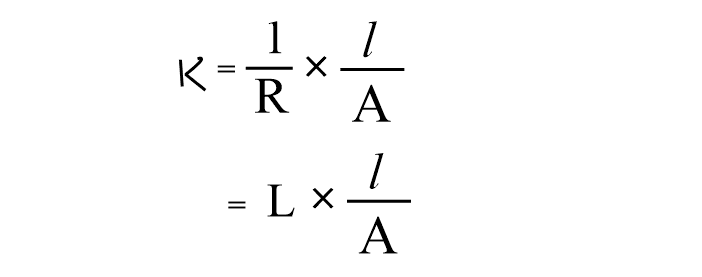
where, L= 1/R,= conductance, which can be defined as the power of electrolytes to conduct electric current.
When l=1cm and A= 1cm2 , we have
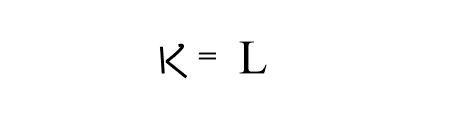
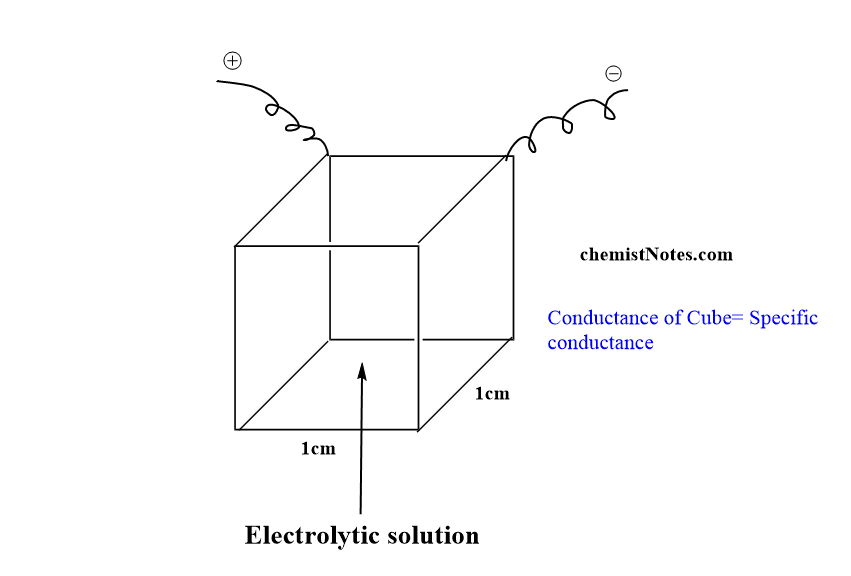
Specific conductance of an electrolytic solution is defined as the conductance of a solution placed between two parallel electrodes which are 1 cm distance apart and have a 1 square cm area of cross-section. In another word, specific conductance is the conductance offered by a one cm cube of a solution.
Specific conductance is an additive property. if more than one electrolytes are present together in a dilute solution, the total specific conductance or conductivity is equal to the sum of specific conductances of individual electrolytes.
- The value of specific conductivity depends on two factors viz. ionic concentration and speed of ions.
specific conductance formula
From the above expression, we have the formula for specific conductance as shown:
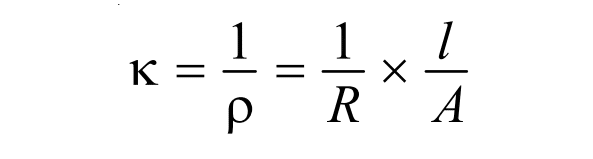
where, R= Resistance, l= length and A= cross-sectional area
This equation can be modified in terms of cell constant. The ratio of specific conductance to that of conductance is called the cell constant.
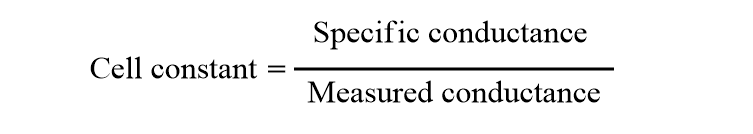
The factor (l/A) is called the cell constant. Hence, the Cell constant is the ratio of the distance between the electrodes to the cross-sectional area of the electrode for a given conductivity cell.
unit of specific conductance
Specific conductance is generally expressed in reciprocal ohms. Its unit can be derived as:
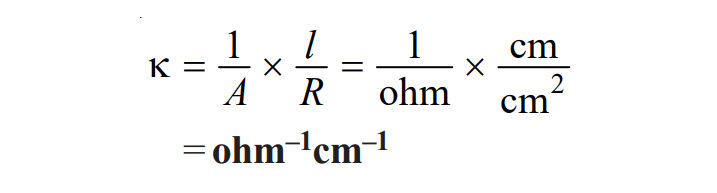
Therefore, the unit of specific conductance is ohm-1 cm-1 or mhos cm-1 or Siemens cm-1 or S cm-1.
Effect of dilution on specific conductance
As already mentioned, the value of specific conductance depends on the number of ions per unit volume of solution. On dilution specific conductance of a solution decreases because the number of ions per unit volume decreases. The lower the number of ions per unit volume, the lower the value of conductivity. Therefore, the specific conductance value increases with an increase in concentration. This can be explained by the fact that in a concentrated solution the ions are not far from each other thus this makes a greater number of ions in unit volume.