Table of Contents
ToggleKohlrausch’s law of independent migration of ions was given by Kohlrausch in 1875. This law is valid for both strong and weak electrolytes, therefore, it is the most important law. This law is based on the concept that at infinite dilution, electrolytes are completely ionized and in this case, the interionic attraction is very low or it can be assumed that there are no such effects.
Kohlrausch’s law
According to Kohlrausch’s law, the sum of the equivalent ionic conductances of ions of an electrolyte gives the equivalent conductance of that electrolyte at infinite dilution. At infinite dilution, ions are far apart from each other and hence interionic attractions are almost absent. This is the reason for the maximum equivalent conductance of electrolytes at infinite dilution.
This law can be stated in another way ” At infinite dilution where dissociation of all electrolytes is complete and where all interionic attractions disappear, each ion migrates independently of its co-ion(partner ions) and contributes to the total equivalent conductance.
Mathematically, Kohlrausch’s law can be expressed as:
λ∞= λ+ + λ–
or λ∞= λc + λa
where, λ∞= equivalent conductance at infinite dilution
λ+ or λc = equivalent conductance of cation at infinite dilution
λ– or λa= equivalent conductance of anion at infinite dilution.
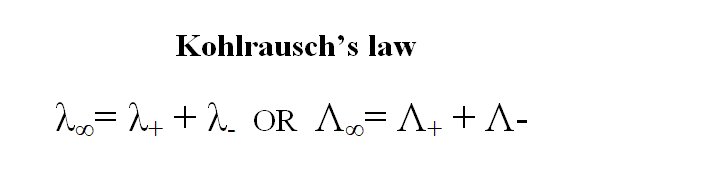
Let us consider an electrolyte AB which dissociates completely at infinite dilution. If Z ohm-1 cm2 eq-1 be the equivalence conductance of AB at infinite dilution, X and Y ohm-1 cm2 eq-1 be the equivalent ionic conductances of cation A+ and anion B- respectively, then according to Kohlrausch’s law:
Z= X + Y
Let’s take the example of NaCl. The equivalent conductance of NaCl at infinite dilution at 25°C is found to be 126.45 ohm-1 cm2 eq-1. The equivalent conductance of Na+ and Cl– ion is 50.11 and 76.34 respectively, then According to the above law, the sum of equivalent conductance of component ions at infinite dilution must equal that of NaCl.
Application of Kohlrausch’s law
Some major applications of Kohlrausch’s law of independent migration of ions are listed below:
- It can be used to calculate the λ∞ value of weak electrolytes. Weak electrolytes do not ionizes completely even at great dilution thus practical determination is impossible. Therefore, in such case Kohlrausch’s law is applicable.
- It can be used to determine the solubility of sparingly soluble salts such as AgCl, PbSO4
- Kohlrausch’s rule is applicable in the determination of degree of ionization of electrolytes and ionic product of water.
References
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and CompanyLtd., New Delhi, 2012
- M. L. Sharma & P. N. Chaudhary, A Textbook of B. Sc. Chemistry (Volume II), 2nd Edition, Ekta Books Nepal, 2007






