Table of Contents
ToggleVariation of conductance with dilution can be explained for both weak electrolytes as well as strong electrolytes. The specific conductances decrease with increasing the dilution while both molar and equivalent conductance increase on dilution. These facts have been discussed below:
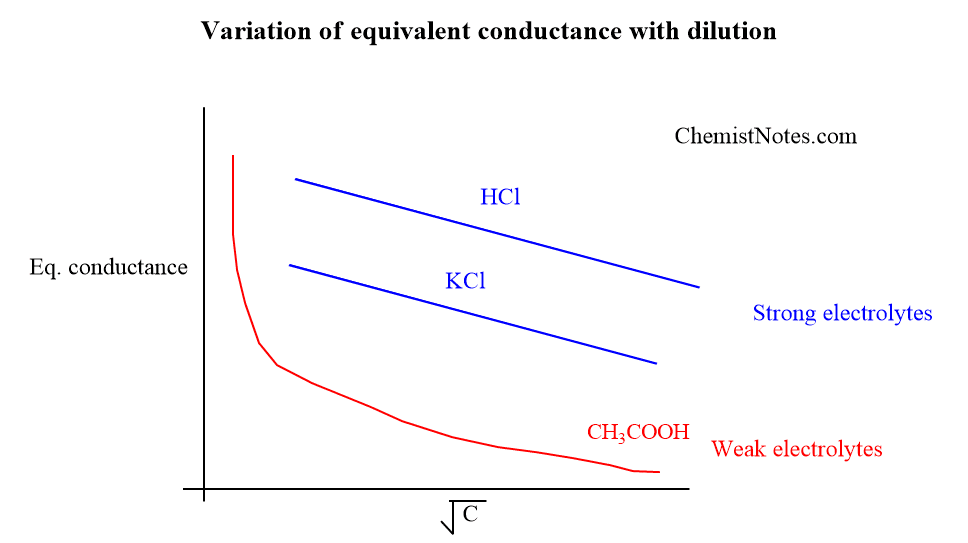
Variation of equivalent conductance with dilution
The equivalent conductance of an electrolytic solution depends on the number of ions and the mobility of ions i.e the speed with which the ions move. On dilution, the number of ions as well as the speed of ions increases. The number of ions increases due to an increase in the degree of ionization and the mobility(speed) of ions increases due to a decrease in interionic attraction between oppositely charged ions on dilution. Therefore, the equivalent conductance increases on dilution.
In the case of weak electrolytes, the degree of ionization increases, and interionic force decreases on dilution so that the number of ions and speed of ions increases which results in an increase in equivalent conductance.
Similarly, In the case of strong electrolytes, the number of ions is the same at all dilutions. The increase in equivalent conductance is mainly due to an increase in the speed of ions with dilution. As dilution is increased, the interionic forces decrease as a result, the speed of ions increases.
Variation of equivalent conductivity with dilution graph
The variation of equivalent conductance or conductivity with dilution can be illustrated graphically for both strong electrolytes and weak electrolytes.
Variation of molar conductance with dilution
The molar conductance of an electrolytic solution depends on the number of ions and the mobility of ions. Therefore, as in the case of equivalent conductance, the number of ions and the speed of ions increases on dilution. The increase in speed is due to a decrease in the interionic interaction between oppositely charged ions. Hence, the molar conductance of both weak, as well as strong electrolytes, increases on increasing dilution or decreasing the concentration.
You may have doubts about the condition of high concentration solution, right? It’s simple, at high concentrations of strong electrolytes, the ions are not far from each other, so the interionic interaction between them is high, which renders the mobility of ions. Consequently, the molar conductance decreases with an increase in concentration.
Variation of specific conductance with dilution
As we know, specific conductance depends on the number of ions per unit volume of solution. On increasing the dilution or on decreasing the concentration, the number of ions per unit volume decreases so that the specific conductance of an electrolytic solution goes on decreasing with dilution. This can be explained in two cases.
In the case of weak electrolytes, although the degree of ionization of electrolytes increases on dilution, the number of ions per unit volume of the solution decreases on dilution because of increased distances between the ions so that specific conductances decrease.
Similarly, in the case of strong electrolytes, the number of ions per unit volume goes on decreasing on dilution. Therefore, the specific conductance of strong electrolytes, as well as weak electrolytes, decreases on increasing dilution or on decreasing concentration. It means specific conductance increases with increases in the concentration of an electrolytic solution.
Variation of conductance with dilution video
References:
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012






