Table of Contents
ToggleA fuel cell is a cell that converts the chemical energy of a fuel directly into electrical energy through a redox reaction. These are primary cells in which fuel such as H2, CO, CH4, C3H8, alcohol, etc. are used to generate electrical energy without the involvement of thermal devices like boilers, turbines, etc. When hydrogen is used as a fuel, the only products are electricity, water, and heat. Fuel cells can create power indefinitely as long as fuel and oxygen are available. Materials to be oxidized and reduced at electrodes are kept outside the cell and continually supplied to the electrodes in fuel cells.
Types of fuel cells
A fuel cell consists of two electrodes a positive electrode (cathode) and a negative electrode (anode) sandwiched around an electrolyte. Fuel is fed to the anode, and the air is fed to the cathode. Depending upon the kind of fuel used, fuel cells are of the following types:
- Hydrogen-oxygen fuel cell
- Hydrocarbon-oxygen fuel cell
- Methyl alcohol-oxygen fuel cell
- CO-oxygen fuel cell
Hydrogen-oxygen fuel cell
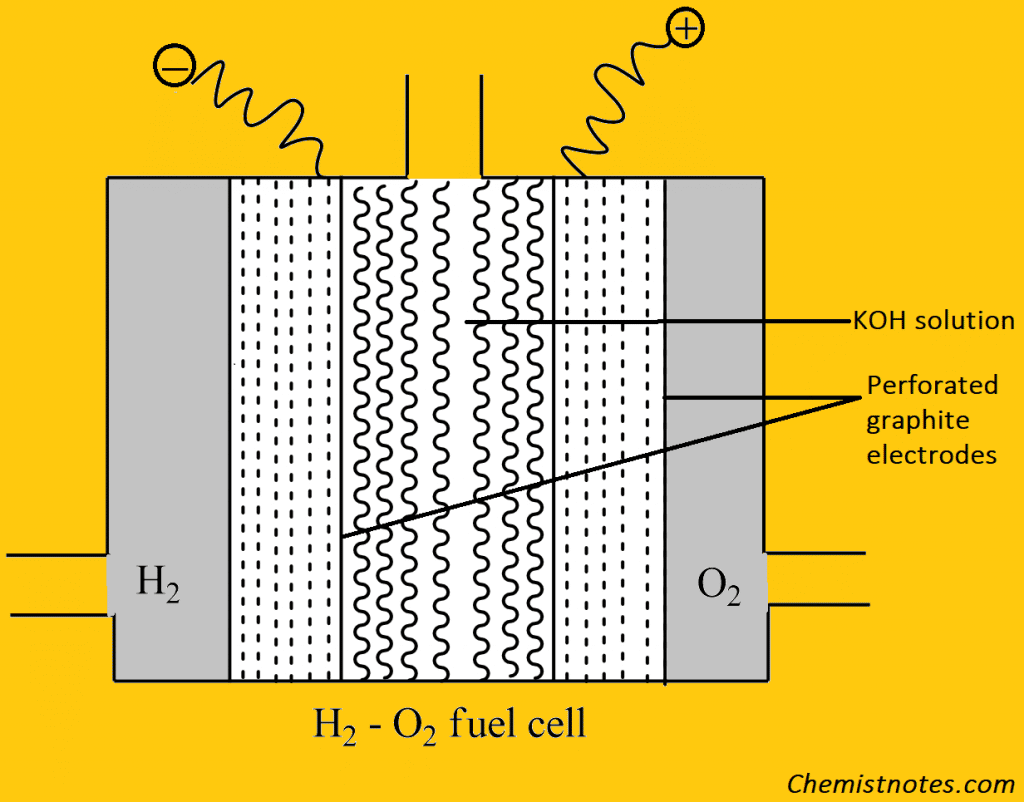
It is the most successful fuel cell and consists of two electrodes made of perforated graphite which are coated with Pt or Ag, which act as catalysts. These electrodes are placed in an aqueous solution of NaOH or KOH. Hydrogen and oxygen are passed into the cell under a pressure of 50 atm and 251 oC. The electrode reaction of the cell is:
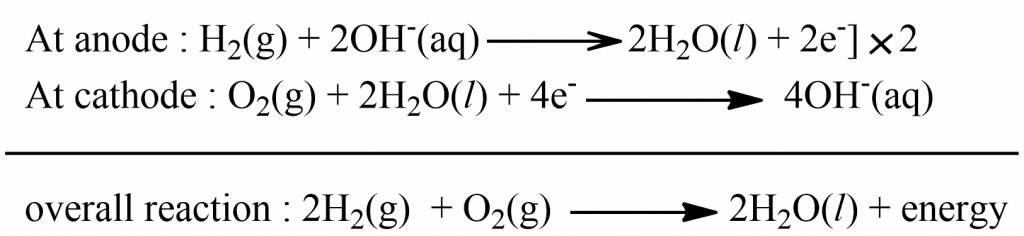
Some important points
- Fuel cells can be used to provide power for a variety of uses, including transportation, industrial/commercial/residential structures, and long-term power storage for the grid in reversible systems.
- The fuel cell has high efficiency. Theoretically, it has above 95% efficiency, but practically it possesses more than 80% efficiency.
- The fuel cell generates power of 1 KW to 100 KW. It has pollution-free working and acts as a continuous source of energy.
FAQs
What is fuel cell?
A fuel cell is a cell that converts the chemical energy of a fuel directly into electrical energy through a redox reaction.






