Table of Contents
ToggleThe law of mass action is a qualitative relationship between the rates of reaction of species. It was proposed by two Norwegian chemists, Guldberg and Waage. It states that “the rate at which a substance reacts is directly proportional to its active mass, and the rate of a chemical reaction is directly proportional to the product of the active masses of the reactions.”
The term “active mass” or “molecular concentration” of a substance stands to indicate the number of gram moles of the substance dissolved in per litre solution and is repressed by enclosing the symbols or formulae of the substance in square brackets.
Examples of the law of mass action
The active mass of A is represented as [A] or sometimes by CA. Let us consider a reversible reaction.
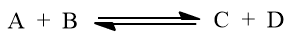
According to law of mass action,
Rate of forward reaction α [A] [B]
or, rate of forward reaction = Kf [A] [B]
where, [A] and [B] are the active mass of reactants A and B respectively, Kf is a rate constant for the forward reaction.
Similarly, rate of backward reaction = Kb [C] [D]
where, Kb is the rate constant for the backward reaction and [C] and [D] are active mass or molar concentrations of products C and D respectively.
At equilibrium, rate of forward reaction = rate of backward reaction
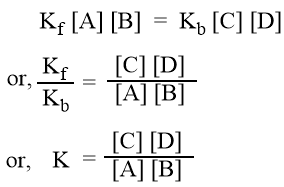
where, K is a constant which is called equilibrium constant. This is the ratio of velocity constants of forward and backward reactions.
What affect the value of equilibrium constant?
- Not the initial concentration or concentration change affect the value equilibrium constant
- Not any catalyst change the equilibrium constant
- Only the temperature affect the value of equilibrium constant
Let us take another type of reaction
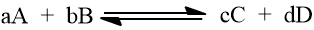
The expression of equilibrium constant can be written as
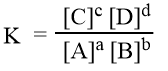
The equilibrium constant, K is defined as the ratio of product of the equilibrium concentrations of the products to that of the reactants with each concentration term raised to the power equal to the stoichiometric coefficient of the substance in the balanced chemical equation.
Hence, the law of mass action can more appropriately be stated as; the rate of a reaction is directly proportional to the product of active masses of reactants, with each concentration term raised to the power equal to the numerical coefficient of that species in the chemical reaction.
Application of Law of mass action
- Law of mass action is used to predict direction of reaction.
- Law of mass action is used to extend of reaction.
FAQs
What is law of mass action?
Law of mass action states that “the rate at which a substance reacts is directly proportional to its active mass, and the rate of a chemical reaction is directly proportional to the product of the active masses of the reactions.”







2 Responses
I surfed many websites on the internet, but did not found such a basic details of physical chemistry, but here. Thanks to chemistnotes.com.
Thank you very much.