Table of Contents
ToggleZaitsev rule, also popularly known as the Saytzeff rule, is a rule that predicts the regioselectivity of olefin formation or the orientation of the carbon-carbon double bond by the elimination reaction of secondary or tertiary alkyl halides or alcohols.
Zaitsev rule
The dehydrohalogenation of the following substrate have only one kind of β-hydrogen, thus yielding only a single elimination product.
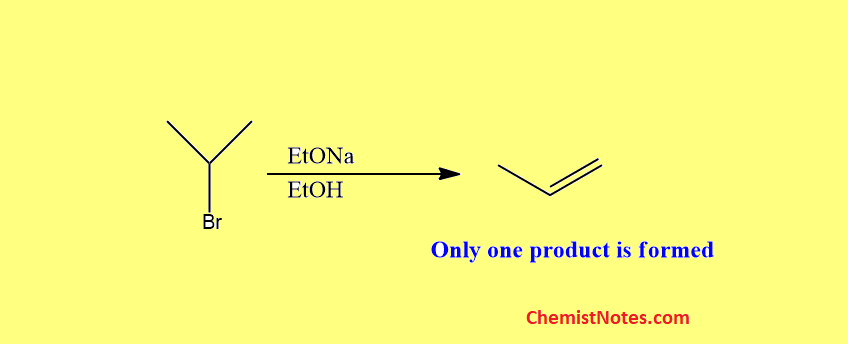
But the dehydrohalogenation of the substrates having different kinds of Beta-Hydrogens yields more than one elimination product.
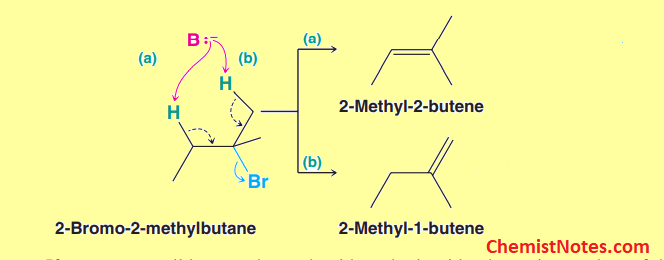
In such cases, a more substituted alkene is generally observed to be the major product. This pattern was originally noticed in 1875 by Russian chemist Alexander M. Zaitsev, who devised the Zaitsev rule. According to this rule, the more substituted alkene is formed as a major product if there is no steric hindrance.
What is Zaitsev’s rule?
This rule states that the proton is preferentially removed from the carbon atom with the fewest protons to form a more stable olefin. In accordance with this rule, the elimination of halides, alcohol, esters, etc. typically produces thermodynamically stable compounds. This type of elimination is referred to as Zaitsev elimination, and the associated stable product is known as a Saytzeff product or Zaitsev product.
If we use a small base such as ethoxide or hydroxide, the major product of the reaction will be the more highly substituted alkene which is also the more stable alkene.
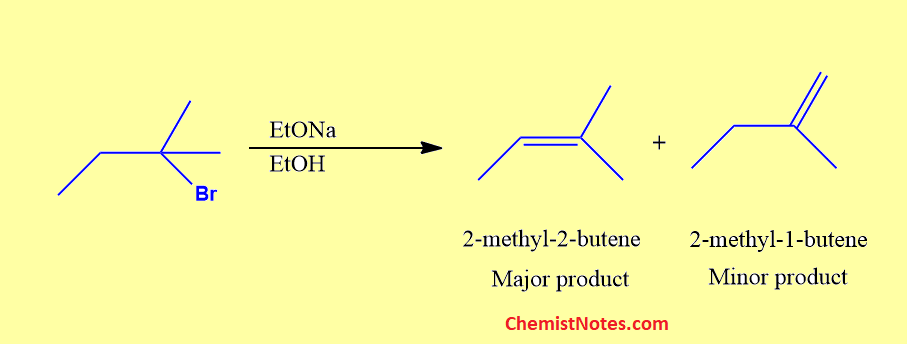
Here, 2-methyl-2-butene is a trisubstitued alkene.Thus, 2-methyl-2-butene is the major product.
The reason for this behavior can be explained on the basis of the double-bond character that develops in the transition state. In the transition state, a double bond character is developed. The more stable transition states are those that resemble a more stable alkene. Hence, the more stable the T.S, the free energy of activation for this reaction is lower and formed faster.
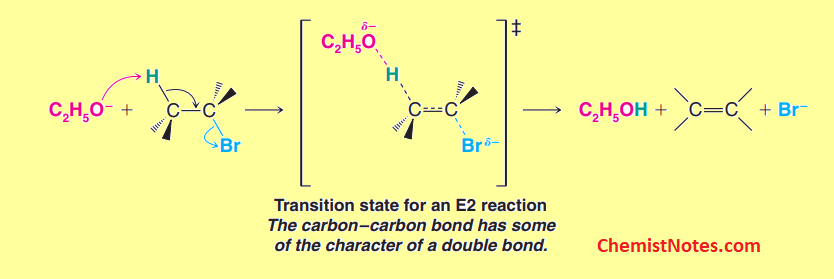
Note:
- This rule does not apply to the pyrolysis of esters in the gas phases and the elimination of onium salts( Quaternary ammonium salts and substituted sulfonium salts). Such a reaction follows the Hofmann rule which states that the least substituted olefins predominate in the products.
- Chugaev reaction and pyrolysis of esters in the liquid phase also follow the Saytzeff rule.
- The steric influence during the elimination process may cause the product to follow the Hofmann rule.
- Hence, the size of the base used in the elimination also influences the regioselectivity of the Saytzeff and Hofmann rule.
Markovnikov rule vs Zaitsev rule
Markovnikov’s rule is used to determine the major product when an unsymmetrical alkene undergoes an electrophilic addition reaction while Zaitsev rule is used to determine the major alkene product when the substrate such as alkyl halide undergoes an elimination reaction. Therefore, simply Markovnikov’s rule is for addition reaction while Zaitsev’s rule is for elimination reaction.
According to Markovnikov’s rule, “When an unsymmetrical reagent is added to an unsymmetrical alkene, the negative part of the reagent is added to the unsaturated carbon having less number of hydrogen atoms.” On the other hand, according to Zaitsev’s rule, the elimination of the substrate occurs in such a way that the more substituted alkene is formed as a major product.
Hofmann rule
When either both substrates and the base are sterically hindered or only one of them is sterically hindered, the less substituted alkene is often the major product during the elimination reaction. This rule is known as Hofmann’s rule and the product formed is called the Hofmann product. Hofmann’s rule is also known as Anti-Zaitsev’s rule.
The relative ratio of Zaitsev and Hofmann products depends on a number of factors so it is difficult to predict. Mainly, the choice of base ( how sterically hindered the base is) plays an important role.
Let’s consider the following example.
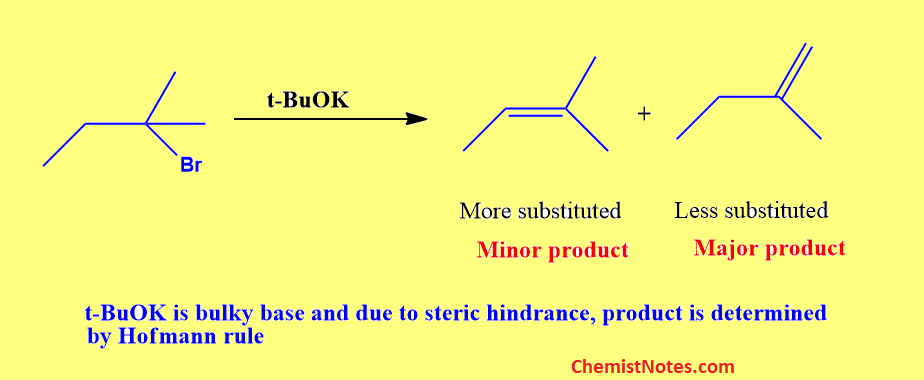
Here, the t-BuOk base is bulky and it is associated with solvent molecules and thus made even bulky. Because of the increased crowding at the transition state, the big tert-butoxide ion appears to have difficulties removing one of the internal hydrogen atoms. Thus, Only one of the methyl group’s hydrogen atoms that is more exposed is removed.
Zaitsev vs Hofmann rule
The major difference between Zaitsev and Hofmann rules is that the Zaitsev rule states that the most substituted alkene is the most stable product, whereas Hofmann’s rule indicates that the least substituted alkene is the most stable product.
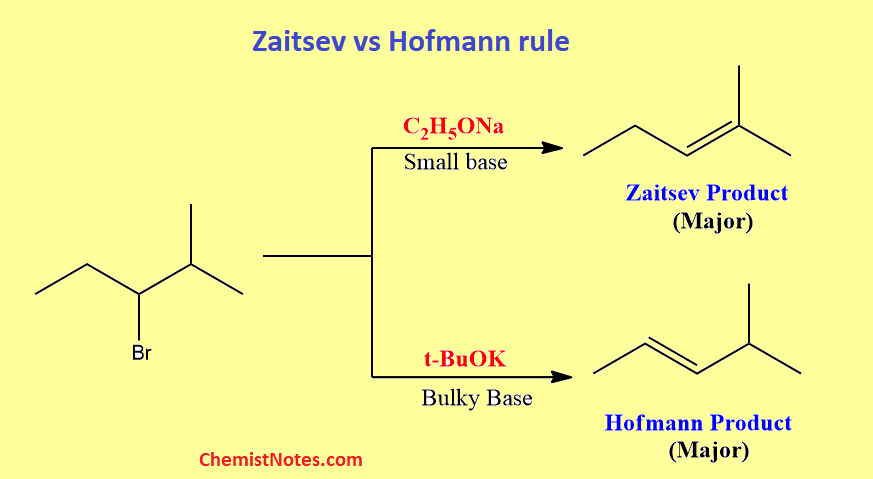
| Zaitsev’s rule | Hofmann’s rule |
| According to it, highly substituted alkene is formed as the major product. | According to it, the least substituted alkene is formed as the major product. |
| When the small base is used, then elimination follows this rule. | When the bulky base is used, then elimination follows this rule. |
Application of Zaitsev rule
This rule is very useful in predicting the regioselectivity of the elimination reaction, which means it is useful for determining the direction of the double bond in the elimination reaction.
FAQs/MCQs:
Does E1 always follow Zaitsev’s rule?
Yes, E1 always follows the Zaitsev rule if there is no Bulky base. If a bulky base is used, there would be a steric hindrance and hence follows the anti-Zaitsev rule( Hofmann’s rule).
When is Zaitsev’s rule not followed?
When there is steric hindrance then the Zaitsev rule is not followed.
Zaitsev rule organic chemistry video
References:
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- R. T. Morrison & R. N. Boyd, Organic Chemistry, 6th and 7th Edition, Prentice- Hall of India Pvt., Ltd., 2008.






