Table of Contents
ToggleMarkovnikov’s rule is followed to determine the major product when unsymmetrical alkene reacts with unsymmetrical reagents such as hydrogen halide.
Alkene on reacting with hydrogen halide like HCl, HBr, HI gives alkyl halide. If the alkene is symmetrical then only one product is formed. If the alkene is unsymmetrical then more than one product is formed. Therefore, the addition of hydrogen halide onto unsymmetrical alkene follow this rule.
Markovnikov’s rule
Markovnikov’s rule states that, “When an unsymmetrical reagent is added to an unsymmetrical alkene, the negative part of the reagent is added to the unsaturated carbon having less number of hydrogen atoms.”
Examples of Markovnikov’s rule
Propene reacts with HBr to give 2-bromopropane as the major product.
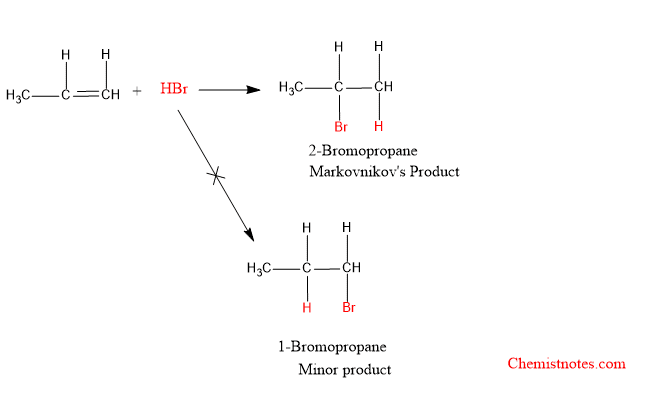
In the above reaction, the carbon atom of 2-position has less number of hydrogen atoms. According to this rule, the negative part of HBr, Br– is added to the carbon atom of the 2-position. Therefore, the major product is 2-Bromopropane instead of 1-Bromopropane.
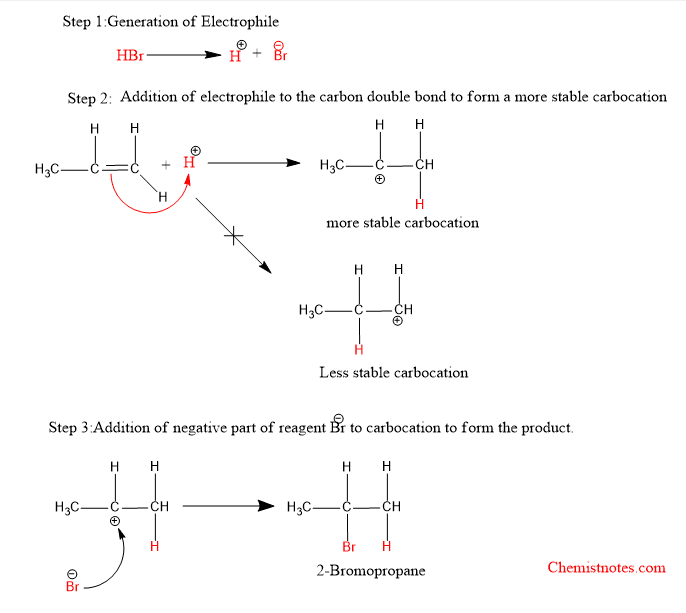
Mechanism of Markovnikov’s rule
The addition of hydrogen halide to unsymmetrical alkene follows this rule. This reaction is electrophilic addition. The mechanism of this reaction is shown below:
What is Anti-Markovnikov’s rule?
Anti-Markovnikov’s rule states that “When HBr is added to an unsymmetrical alkene in presence of traces of organic peroxide, bromine is added to the unsaturated carbon having more hydrogen atoms“. This rule is also called the peroxide effect or the Kharash effect.
This rule is opposite to Markovnikov’s rule. Therefore, the product formed is against to Markovnikov rule. This reaction takes place only in presence of organic peroxide, hence it is also called the peroxide effect.
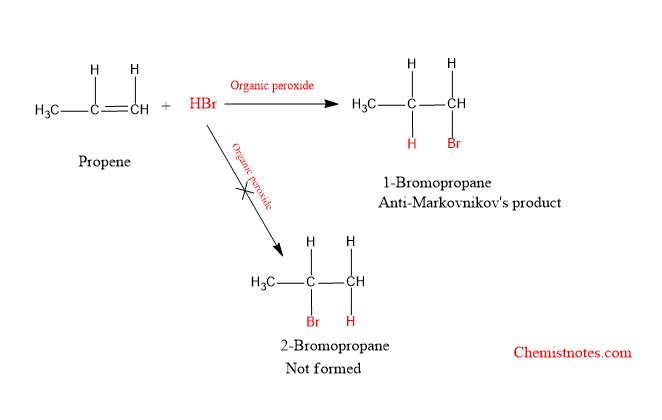
Example of Anti-Markovnikov’s rule
When propene reacts with HBr in presence of organic peroxide, 1-bromopropane is formed as the major product.
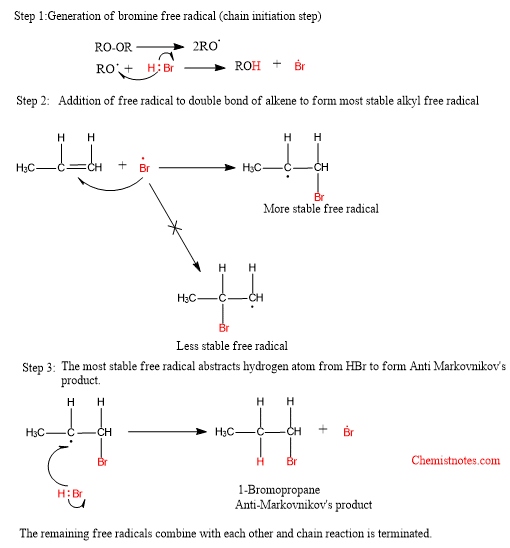
Mechanism of Anti-Markovnikov’s rule
The addition of HBr to an unsymmetrical alkene in presence of organic peroxide follows this rule. This reaction is a free radical addition reaction and proceeds via a free radical mechanism. The mechanism of peroxide effect is illustrated as:
Why Peroxide effect is shown by HBr and not by HCl or HI?
This can be explained on the basis of the homolytic bond dissociation energy of HCl, HBr, and HI. H-Cl bond has greater bond dissociation energy(103Kcal/mol) and it is a stronger bond so that the H-Cl bond is not cleaved easily by alkoxy free radical generated by organic peroxide.
Similarly, the H-I bond has the lowest bond dissociation energy(71Kcal/mol) and it is the weakest bond. H-I bond is cleaved easily by alkoxy free radical to form Iodine free radical but these iodine free radical combines with each other to form iodine molecule instead of attacking the double bond of an alkene. Therefore, the peroxide effect is shown only by HBr but not by HCl and HI.
FAQs/MCQs:
What is anti markovnikov’s rule?
When HBr is added to an unsymmetrical alkene in presence of traces of organic peroxide, bromine is added to the unsaturated carbon having more hydrogen atoms. This is called Anti-Markovnikov’s rule.
What is markovnikov’s rule?
According to this rule, “When an unsymmetrical reagent is added to an unsymmetrical alkene, the negative part of the reagent is added to the unsaturated carbon having less number of hydrogen atoms.”
Does acid halogenation follow markovnikov’s rule?
Yes, acid halogenation follows this rule.






