Table of Contents
ToggleIn 1890, Curtius published the first report of the Curtius Rearrangement reaction. It involves the thermal decomposition of acyl azides to produce isocyanate. The Lossen reaction and the Hofmann rearrangement are both closely connected to this reaction. All of these reactions allow for the production of amines via the synthesis of an isocyanate intermediate.
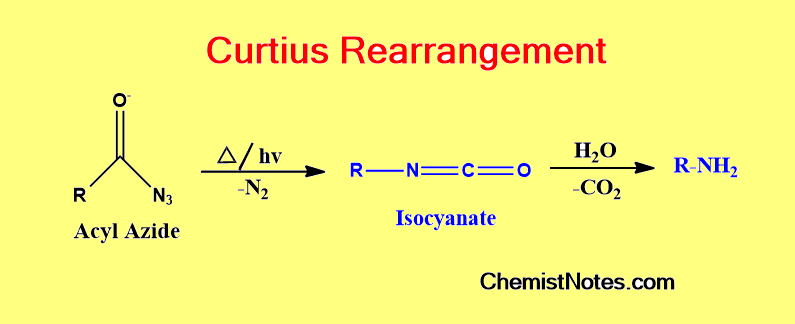
Curtius rearrangement
Curtius rearrangement is the thermal breakdown of an acyl azide to produce an isocyanate by losing N2. The most important modification of Curtius rearrangement is to use the diphenyl phosphoryl azide (dppa), which is known as the Shiori reagent.
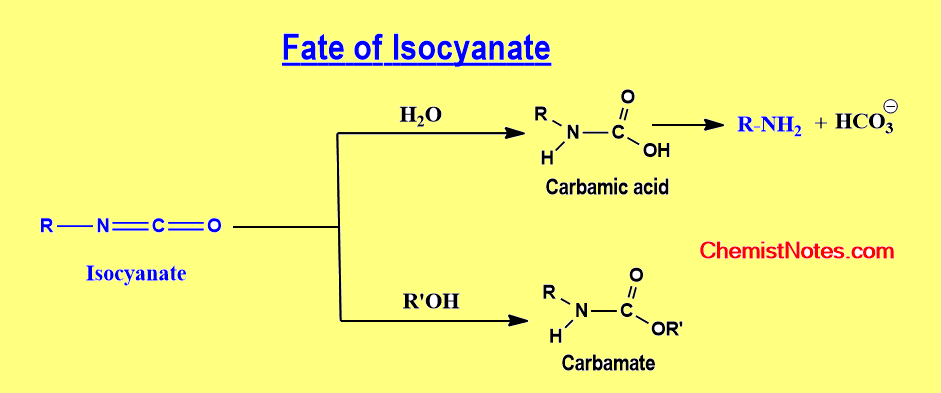
Depending on the reaction conditions, an isocyanate produced during the Curtius rearrangement might undergo a variety of following reactions.
- Isocyanate interacts with water in an aqueous solution to form carbamic acid, which rapidly decarboxylates to form an amine. This is generally referred to as the Curtius Reaction.
- The isocyanate reacts to produce a carbamate when alcohol is employed as the solvent.
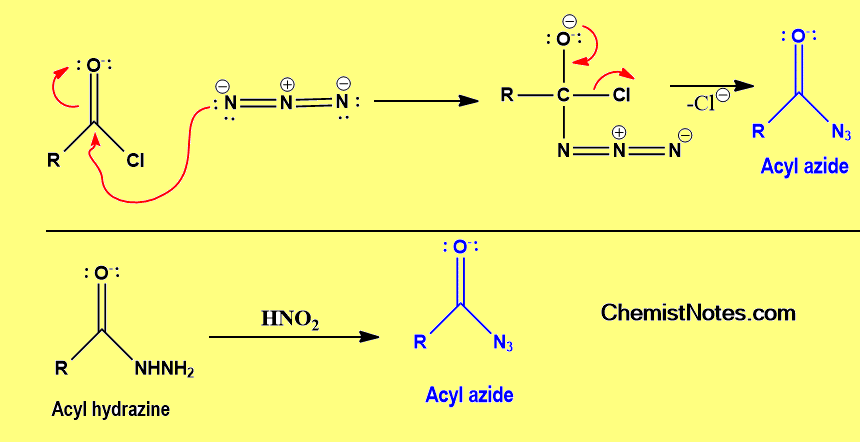
How is acyl azide prepared?
There are many methods of preparation but here we have mentioned common two methods. One can prepare acyl azide from the acyl chloride by treating it with azide ion or sodium azide. Alternately, acyl azide is prepared by treating acyl hydrazine with nitrous acid.
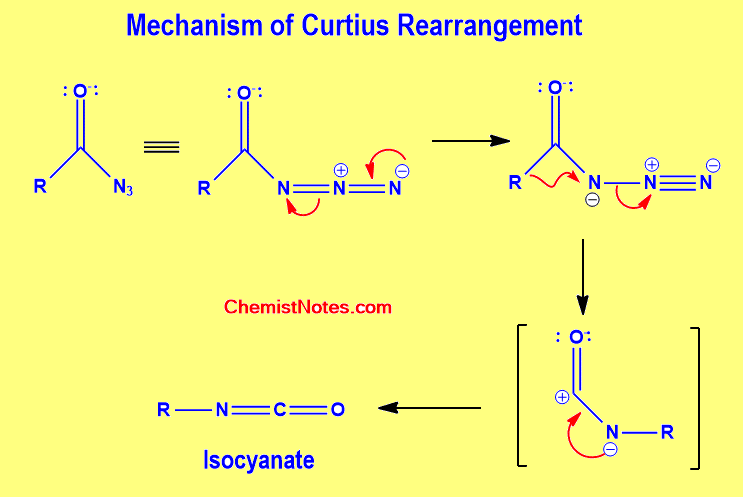
Curtius rearrangement mechanism
According to reports, this reaction proceeds via a concerted mechanism, producing a carbonyl nitrene intermediate from which the alkyl group migrates to the nitrogen atom to produce an isocyanate intermediate.
Curtius rearrangement applications
- The synthesis of non-commercially available isocyanates from carboxylic acids has been achieved using the Curtius rearrangement.
- This rearrangement is useful for producing tetrazoles, cyanamides, amino alcohol, and Furano carbamate.






