Table of Contents
ToggleYlides are dipolar compounds in which a carbanionic carbon is immediately adjacent to an atom bearing a positive center. This blog will deal with ylids with their types, stability, and their generation.
Ylides
Ylides are defined as compounds in which a carbanion is directly bonded to a positively charged heteroatom such as Phosphorus, Sulphur, Nitrogen, etc. In other words, ylides are made up of two oppositely charged atoms of different elements that are next to one another.
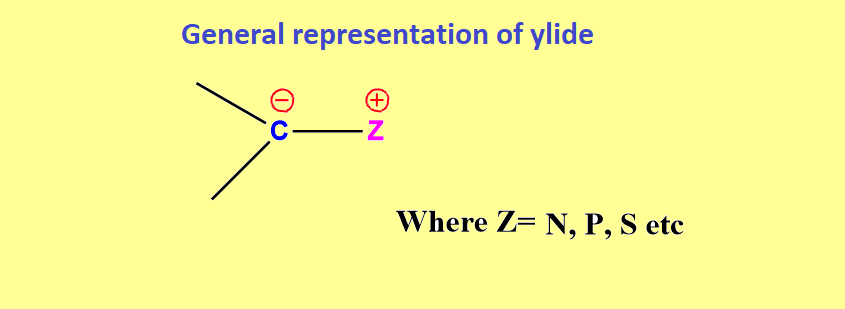
These reagents are nucleophilic at anionic carbon atoms while electrophilic at cationic heteroatoms.
Types of Ylide
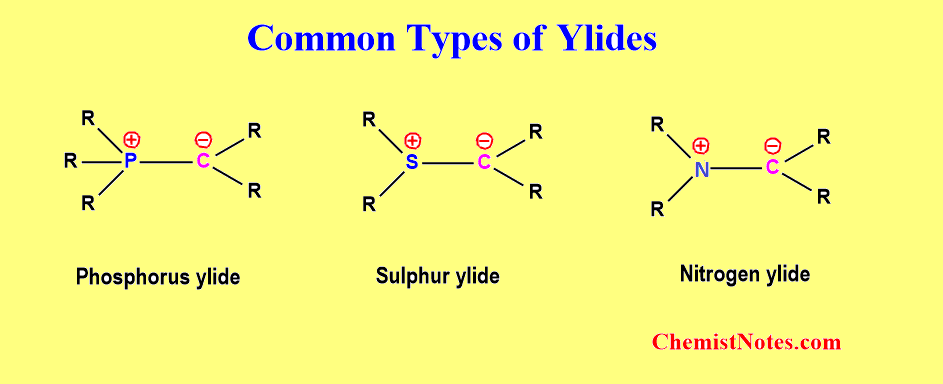
There are various types of ylides but the most common types are given below.
- Phosphorus ylide
- Sulfur ylide
- Nitrogen ylide
Of these three classes of ylides, phosphorus ylide is a widely studied compound due to its stability and ability to generate alkene from carbonyl compounds. Sulfur ylids are less reactive than phosphorus ylides, and are well known to form cyclopropane derivatives with carbonyl compounds. In contrast, nitrogen ylides are very unstable and thus, have very little synthetic utility.
Stability of Ylides
The stability of ylides depends greatly upon two factors viz extent of pπ-dπ bonding between the filled orbital of a carbon atom and the empty d-orbital of heteroatom and the substituents attached to heteroatom and carbon.
The stability of ylides depends upon the extent of Pπ-dπ bonding between the filled orbital of carbon and the empty d-orbital of heteroatom. The greater the extent of pπ-dπ bonding, the greater the stability and the higher the selectivity.
Moreover, the substituents also play important role in the stability of ylides. As the electron-donating ability of the substituents bonded to the heteroatoms increases, the stability of the ylide increases, and its reactivity decrease.
Similarly, as the electron-withdrawing ability of substituents bonded to the carbanion carbon increases, the stability of the ylides increases, and its reactivity decreases.
The participation of d-orbital in pπ-dπ bonding for phosphorus is greater than that of sulfur, so phosphorus ylids have higher stability hence more selective than sulfur ylides.
Stabilized vs Unstabilized ylide
The major difference between stabilized and unstabilized ylide is that there is resonance stabilization due to the presence of suitable substitution on the carbon atom in stabilized ylide but such groups are absent in the case of unstabilized ylide.
Simply, we can take an example of phosphorus ylide.
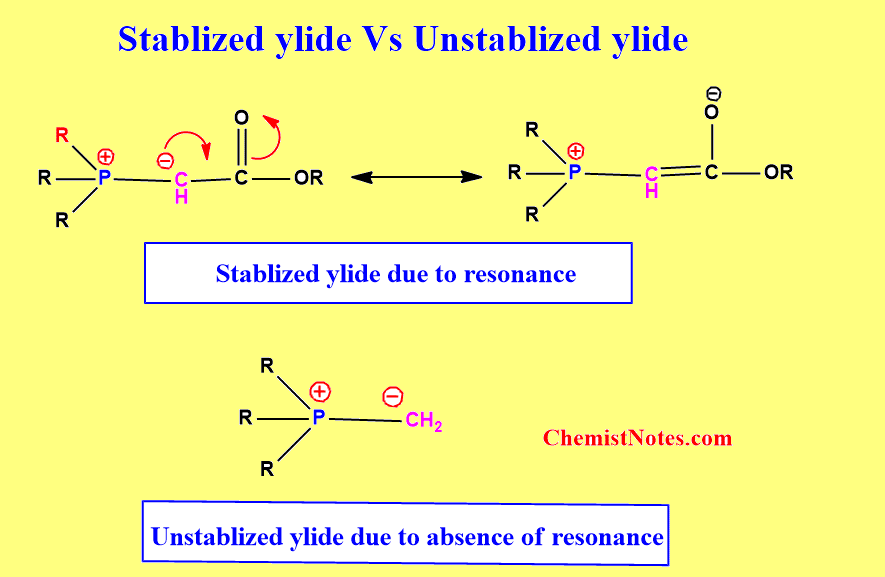
In this example, you can see that there is a substituent on the C-atom that can stabilize the
negative charge by delocalization(Resonance)
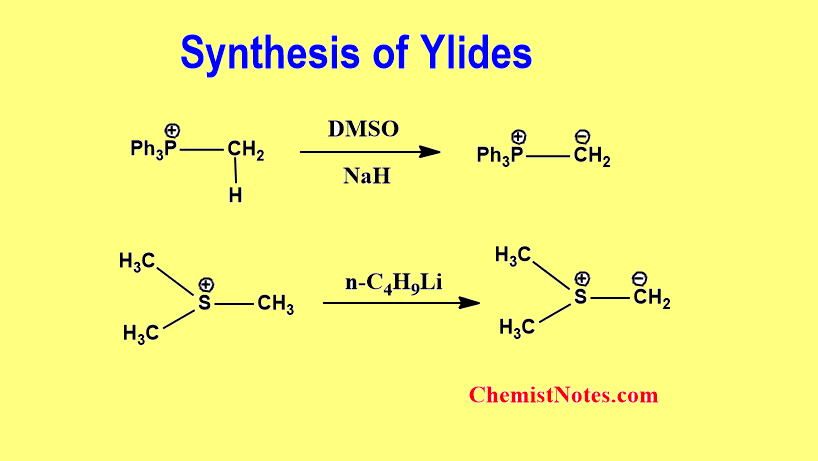
Ylide synthesis
The method of ylide synthesis varies from one type to another type of ylide. It means, there are different methods of preparation, which depend on the type of ylid. Phosphorus ylides and sulfur ylides are prepared generally from corresponding salts using a suitable base.
FAQs/MCQs:
what are ylides?
These are dipolar compounds in which a carbanionic carbon is immediately adjacent to an atom bearing a positive center.






