Table of Contents
ToggleWoodward Hydroxylation reaction is the modification of the Prevost hydroxylation reaction that involves the treatment of alkenes with iodine and silver acetate in presence of water to form Cis-diols or cis-glycols. American organic chemist Robert Burns Woodward was the first to report on this reaction in 1954. If you find Woodward Prevost hydroxylation in the literature, it indicates the same Woodward hydroxylation reaction. Don’t get confused !!!!
Woodward hydroxylation
The Woodward reaction is popularly known as Woodward cis hydroxylation since it forms Cis-diols. This reaction involves the synthesis of cis-diols(syn-diols) from alkene by the addition of iodine followed by nucleophilic displacement with acetate ion in the presence of water.
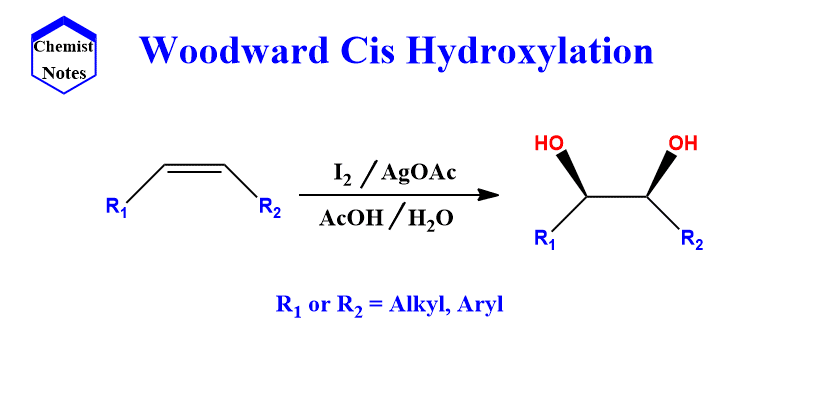
Woodward hydroxylation mechanism
This reaction occurs in two steps, the first of which involves treating olefin with iodine and silver acetate in dry acetic acid at room temperature.
The initial reaction between iodine and olefin results in the formation of a cyclic iodonium intermediate ion, which is then subsequently opened by silver acetate via SN2 substitution to yield α-iodoacetate.
Another SN2 substitution then displaces the iodine to form hydroxylacetate which on hydrolysis produces cis-diol.
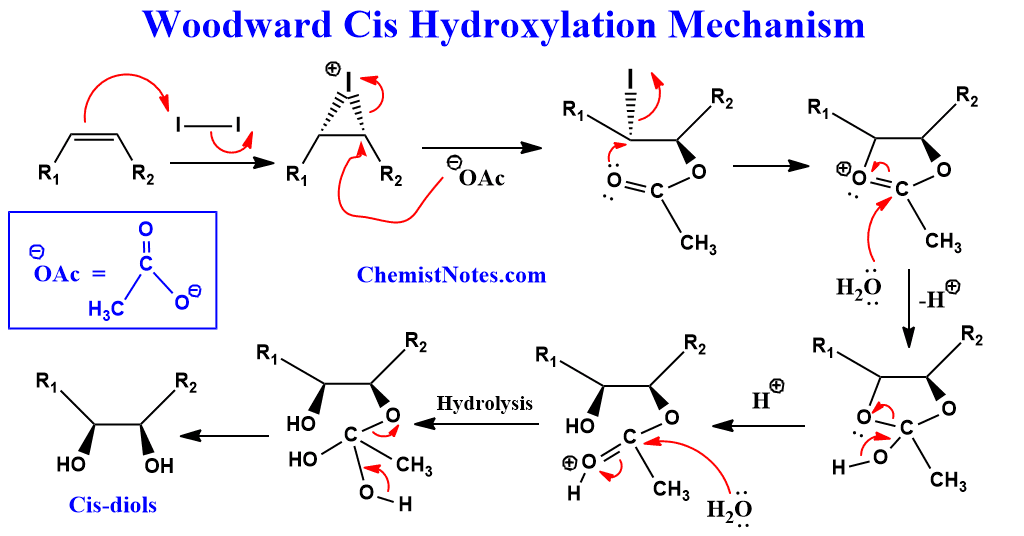
Woodward Cis hydroxylation examples
Let’s see an example of this reaction.
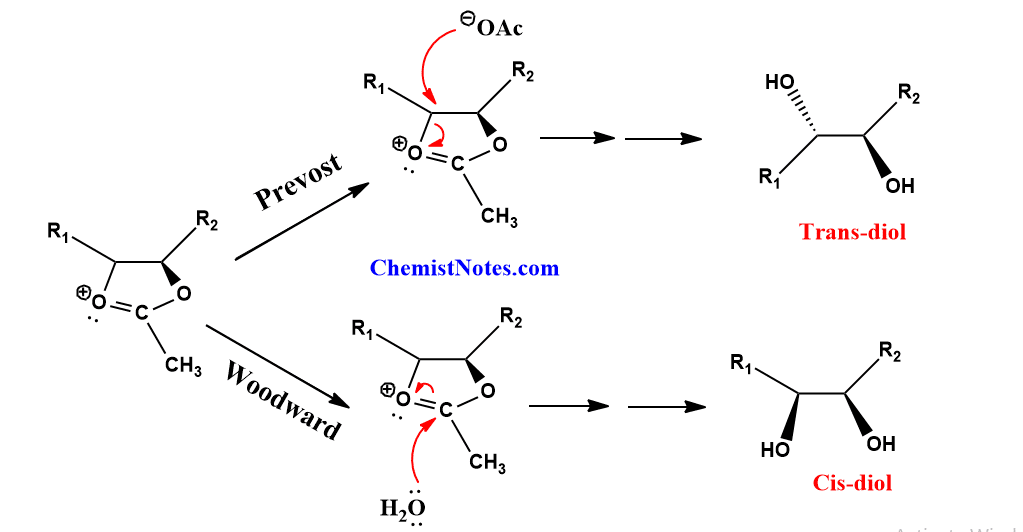
Woodward hydroxylation vs Prevost hydroxylation
The main difference between Woodward and Prevost hydroxylation is that Woodward hydroxylation gives Cis-diol(Syn-diol) whereas Prevost hydroxylation gives trans-diol(anti-diol).
Mechanistically, the first step of both reactions involves the addition of iodine to the carbon-carbon double bond of alkene to form an iodonium intermediate followed by an attack of acetate ion(or benzoate ions), thus α-iodoacetate is formed in both cases. This further undergoes a substitution reaction to form a five-membered cyclic intermediate.
Now, the main difference appears here. In the case of the Woodward reaction, water acts as a nucleophile and adds to the partially positive carbon atom of the five-membered intermediate so that ring is opened. Whereas in the Prevost reaction, acetate ion or benzoate ions open the five-membered ring intermediate as shown below.
Application of Woodward hydroxylation
As stated above, the major synthetic utility of this reaction is the production of cis-diols from alkenes. Moreover, this reaction is a stereoselective reaction hence it can be used in the synthesis of natural products, dyes, pharmaceuticals, etc.
References:
- Woodward, R. B.; Brutcher, F. V., Jr. J. Am. Chem. Soc. 1958, 80, 209−211.
- Mergott, D. J. Woodward cis-dihydroxylation. In Name Reactions for Functional Group Transformations; Li, J. J., Corey, E. J., Eds.; John Wiley & Sons: Hoboken, NJ, 2007, pp 327−332. (Review).






