Table of Contents
ToggleThe Prevost reaction is one of the useful reactions for the preparation of Trans-vicinal diols from alkenes. French chemist Charles Prévost was the first to report on this reaction in 1933. This reaction is also known as Prevost trans-dihydroxylation or Prevost anti-hydroxylation because two hydroxyl groups are added to the opposite faces of alkene double bonds.
Prevost reaction
The conversion of alkene into trans-1,2-diol by treating it with silver carboxylate(I) and iodine in anhydrous solvents followed by reduction or simply hydrolysis is known as the Prevost Trans-dihydroxylation reaction. Silver carboxylate in this reaction might be silver acetate(I) or silver benzoate(I).
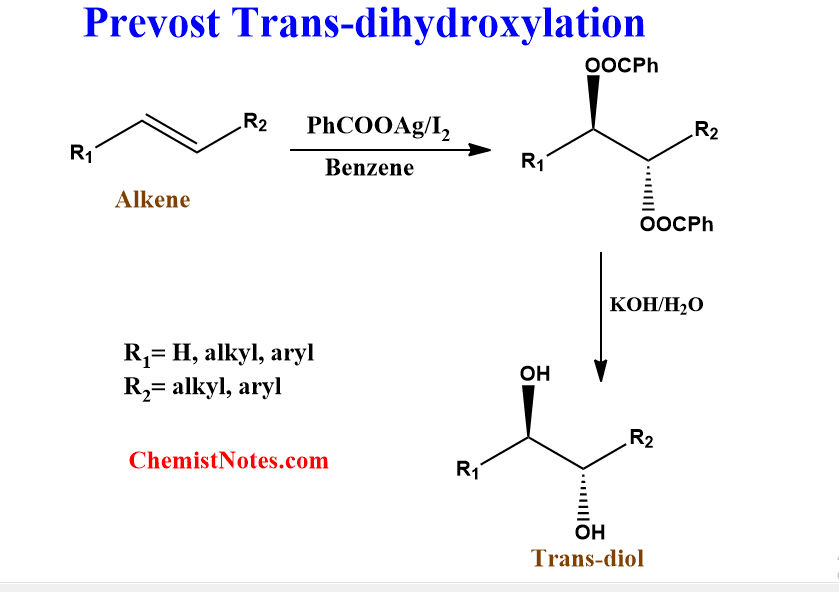
Prevost reaction mechanism
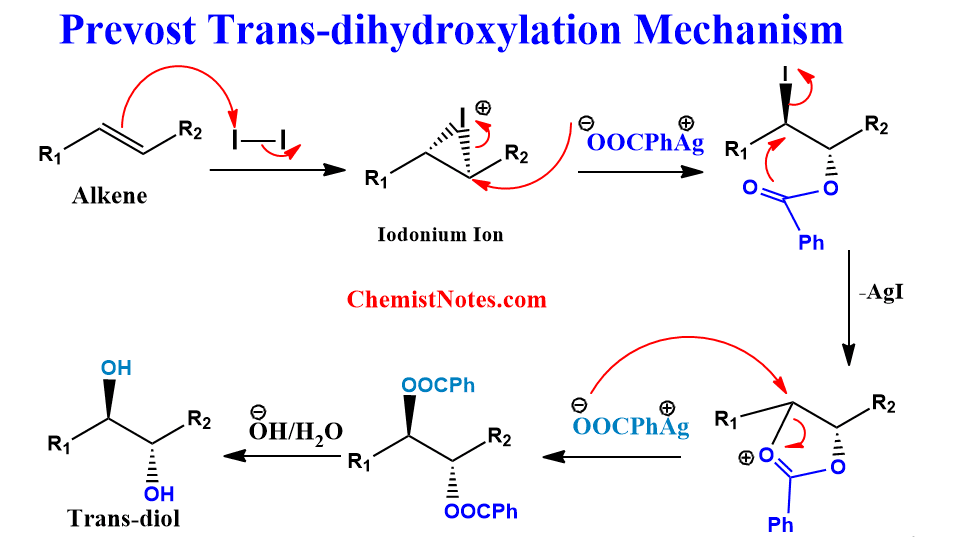
Detailed information on the mechanism of this reaction is not available. However, it is believed that the iodine first adds to the double bond of the alkene to form iodonium cation, similar to the bromination of alkene.
Then, the acetate (or benzoate) ion attacks the iodonium cation nucleophilically to form trans-α-iodoacetate, which immediately loses α-iodo to form dioxoanyl carbocation. This carbocation is further attacked by a second acetate anion to give trans-1,2-diacetate. Finally, trans-1,2-diacetate upon hydrolysis or reduction gives trans-1,2-diol.
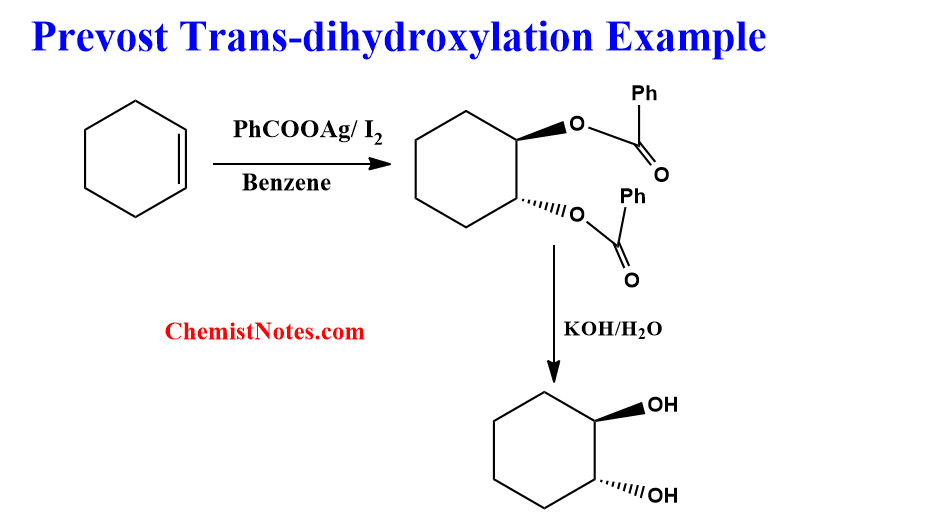
Example of Prevost reaction
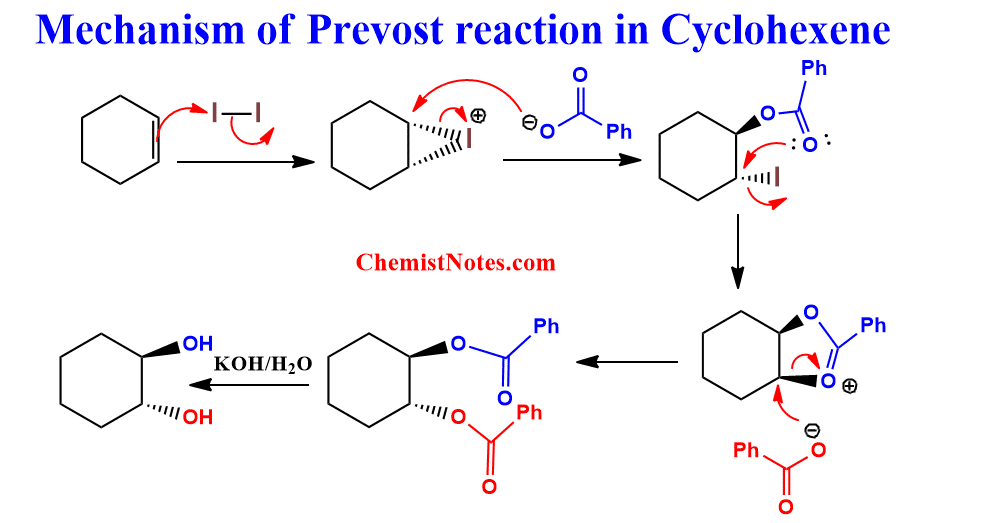
Let’s see an example of cyclohexene undergoing Prevost reaction with the mechanism.
Mechanism:
Application of Prevost reaction
This reaction is popular for the conversion of alkene to trans-diols. Hence, only trans-diols are formed by using this reaction. If we desire to prepare cis-diols from an alkene, there is another reaction known as Woodward Cis-dihydroxylation.
FAQs/MCQs:
What is the difference between Prevost and Woodward hydroxylation reactions?
The Prevost hydroxylation reaction gives trans-diol as a product whereas Woodward hydroxylation gives Cis-diol as a product.
References:
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010.
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009.






