Table of Contents
ToggleIn order to explain the stability of cycloalkane, Baeyer developed a theory known as Baeyer’s strain theory. According to Van Hoff and Le Bel, the four-sigma bond of the carbon atom should be arranged in a regular tetrahedron fashion and hence angle between the two bonds is 109° 28′. If the carbon has a bond angle of 109° 28′, the molecule is free from any strain and the molecule is stable. Baeyer held that any deviation from this tetrahedral angle leads to strain in the molecule resulting in a decrease in the stability of the molecule.
Baeyer’s Strain Theory
The main points of Baeyer’s strain theory are:
- All cycloalkanes are planar or flat i.e. lie in the same plane. Hence bond angles between an adjacent carbon atom of the ring no longer remain 109° 28′. Different rings have different values of this angle. For example, the cyclopropane ring is an equilateral triangle having a C-C-C angle of 60o only.
- Any deviation, positive or negative from the normal tetrahedral bond angle of 109° 28′ during the formation of the ring creates a strain in the molecule which makes the molecule unstable. This deviation of bond angle is known as Baeyer angle strain or simply angle strain.
- The larger (more) the deviation from the normal angle, the greater the strain, and thus lesser the stability. However, it should be noted that the sign of deviation does not make any difference.
- Mathematically, angle strain = 1/2 (109° 28′ – bond angle in planar form)
Relative stability of cycloalkanes
Cyclopropane
In cyclopropane, the three carbon atoms occupy the corners of an equilateral triangle. Thus cyclopropane has a C-C-C bond angle of 60°. This implies that a normal tetrahedral angle of 109° 28′ between any two bonds is compressed to 60o and that each of the two bonds involved is pulled in by the angle strain.
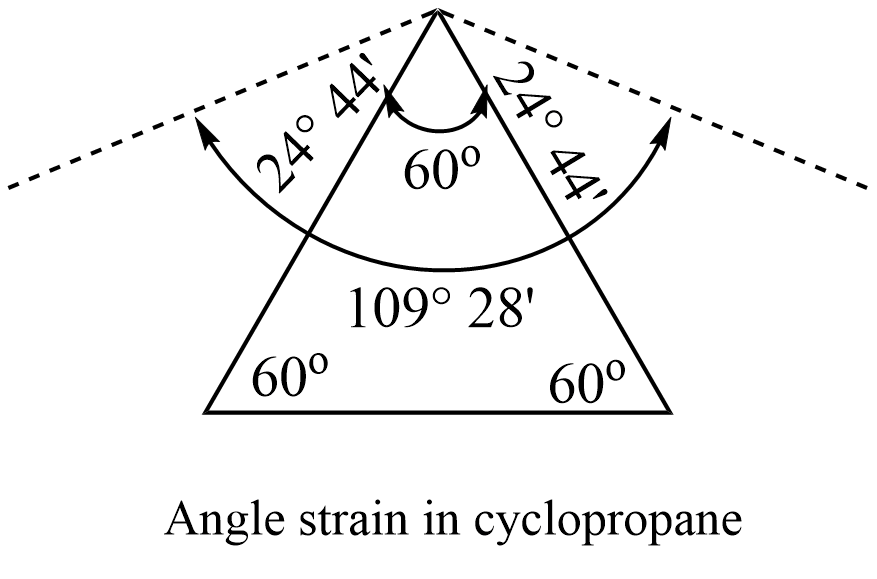
Angle of deviation or angle strain = 1/2 (109° 28′ – 60°) = 24° 44′
The value of 24° 44′ then represents the angle strain through which each bond bends from the normal tetrahedral direction.
Cyclobutane
In cyclobutane, the four carbon atoms occupy the corner of the square. Thus cyclobutane has a C-C-C bond angle of 90°. This implies, that the normal tetrahedral angle of 109° 28′ between any two bonds is compressed to 90°.
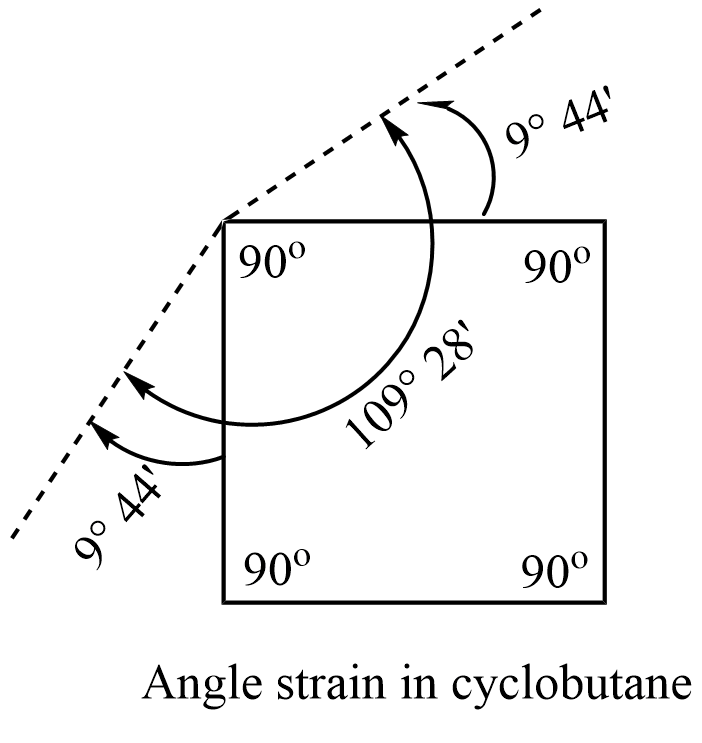
Now, angle strain = 1/2 (109° 28′ – 90°) = 9° 44′
Thus 9° 44′ is the angle strain or angle of deviation through which each bond bends from the normal tetrahedral direction.
Cyclopentane
Bayer assumes that cyclopentane is a planar molecule. So, every C-C-C bond angle becomes 108° like that of a regular pentagon.
Angle strain = 1/2 (109° 28′ – 108°) = 0° 44′
Here, the angle strain of cyclopentane is negligible.
Cyclohexane
Bayer assumed that cyclohexane is a planar molecule. So every C-C-C bond angle becomes 120° like that of a regular hexagon.
Angle strain = 1/2 (109° 28′ – 120°) = – 5° 16′
Therefore, cyclohexane has outward bond angle distortion.
| Cycloalkane | Bond angle | Angle strain |
| Cyclopropane | 60° | 24° 44′ |
| Cyclobutane | 90° | 9° 44′ |
| Cyclopentane | 108° | 0° 44′ |
| Cyclohexane | 120° | – 5° 16′ |
Because of the distortion of the bond angle outwards (1/2 (109° 28′ – 120°) = -5° 16′), cyclohexane is predicted to be unstable by Bayer’s train theory. Cyclohexane is more stable than cyclopentane, as seen by the lower heat of combustion value per -CH2 (157.4 kcal/mol) for cyclohexane. Assuming that all carbon atoms of cycloalkanes with six or more carbon atoms lie in one plane, as Bayer’s strain theory does, causes angle strain in the ring and makes cyclohexane unstable. However, in cycloalkanes with six or more carbon atoms, all of the carbon atoms lie in different planes (puckered), therefore the typical bond angle (109° 28′) is maintained and the ring is not strained.
Drawbacks of Bayer’s strain theory
| Ring size | Heat of combustion per CH2 (kcal/mol) |
| 3 | 166.6 |
| 4 | 164.0 |
| 5 | 158.7 |
| 6 | 157.4 |
| 7 | 158.3 |
| 8 | 158.6 |
From the study of the heat of combustion data, one can easily draw the following conclusion:
- Cyclopropane and cyclobutane release more energy per -CH2– group compared to the open-chain compound. Hence, cyclopropane and cyclobutane are less stable compared to open-chain compounds. It is reasonable to assume that the tendency to undergo ring-opening reactions is connected to this instability. This statement aligns with Baeyer’s strain theory.
- Since Bayer’s strain theory suggests cyclopentane is the most stable and cyclohexane should be unstable because of outward bond angle distortion (-5° 16′). But the heat of combustion value per -CH2– of cyclohexane is 157.4 kcal/mol shows that cyclohexane is more stable than cyclopentane whose heat of combustion value per -CH2 is 158.7 kcal/mol. Hence Baeyer’s strain theory fails to account for the stability of cyclohexane.
- Higher cycloalkanes are also more stable than cyclopentane which is contrary to Baeyer’s strain theory.
So, Baeyer’s strain theory is not applicable for rings larger than cyclopentane because he assumed that the rings are planar or flat. However, these rings are not planar or flat but puckered and each bond angle of carbon is 109° 28′.
Factors affecting stability of conformation of cycloalkane
- Angle strain: Bond angles tend to be coplanar with an atom’s bonding orbitals. Angle strain occurs in molecules when bond angles deviate from the normal bond angle. Cycloalkanes with angle strain have a less stable conformation.
- Torsional strain: When two carbons are bonded together, the bonds tend to be spaced apart. This explains why cycloalkanes like cyclohexane are more stable in their staggered, “chair,” form. When two connected carbons approach an eclipsed state, a torsional strain develops, increasing the energy of the system. Therefore, the staggered conformation has the lowest torsional strain and the eclipsed conformation has the most. Torsional energy is the energy needed to rotate the molecule about the carbon-carbon bond.
- Steric strain: The size and polarity of the groups attached to two linked carbons affect how they interact with one another. These connections can either attract or repel one another. Attractive interactions will exist between the groups or atoms if their distance is exactly equal to the sum of the Vander wall radii. In addition, if these atoms or groups are brought any closer than this, repulsion will set in, and van der Wall strain or steric strain will result.
- Dipole-dipole interactions: Atoms or groups attached to bonded carbons orient themselves to have favorable dipole-dipole interaction. It will be their tendency to have maximum dipole-dipole attractions. Hydrogen bond is a particular case of powerful dipole-dipole attraction.
Baeyer’s strain theory video
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987
- Michael B. Smith, March’s Advanced Organic Chemistry, (7th Edition), John Wiley and Sons, Inc., 2013.
- J. March, Advanced Organic Chemistry, (4th Edition), John Wiley and Sons, 1992






