Table of Contents
ToggleBaeyer’s Strain Theory and its limitations were proposed by Adolf von Baeyer in order to explain the relative stability of various alicyclic rings (aliphatic cyclic rings).
Baeyer’s strain theory and its limitations
Let’s discuss postulates of Baeyer’s strain theory of cycloalkanes first of all, and its limitations later.
Baeyer’s strain theory of cycloalkanes
When a carbon atom is bonded to four other atoms, its four valencies are directed towards the four corners of a regular tetrahedron, and therefore, any two of its bonds form an angle of 1090 28’ the ideal tetrahedral angle at the center of the tetrahedron.
Baeyer’s Strain Theory is based on the following assumptions or postulates.
- An alicyclic ring having all carbon atoms lie in the same plane. Hence, one pair of bonds can not assume tetrahedral angle i.e., 1090 28’.
- Any deviation of carbon-carbon bond angle of the ring form the ideal tetrahedral angle develops a strain in the ring. Such strain is called angle strain which makes the ring unstable.
- Greater the angle strain in the ring, the more unstable is the ring.
- The more stable a ring, more easily it can be synthesized.
The angle strain developed in the ring due to deviation forms regular tetrahedron angle can be expressed in terms of valence angle deviation (d).
d = (1090 28’ – α)/2 where α is the bond angle in the cycloalkane.
For example: Let’s consider cyclopropane ring.
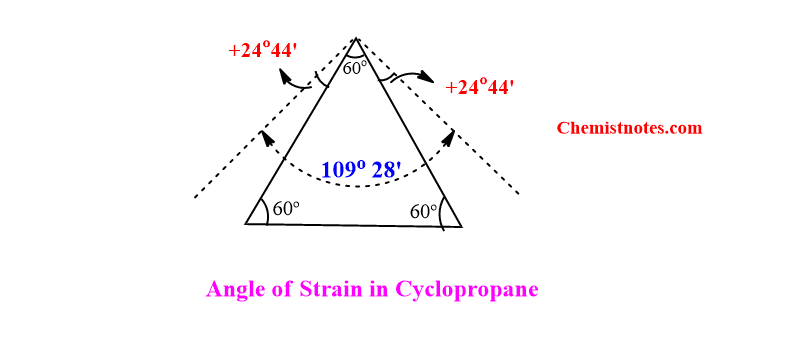
In this ring, the three carbon atoms are situated at the corners of an equilateral triangle. Therefore, the bond angle (α) is 600. This indicates that during the formation of cyclopropane, the regular tetrahedron angle 1090 28’ is compressed to 600.
Now the valence angle deviation (d) =(1090 28’ – α)/2 = 24044’
The angle of strain in cyclopropane is 24044’.Similarly, the angle strain calculated in various ring sizes of cycloalkane can be calculated.
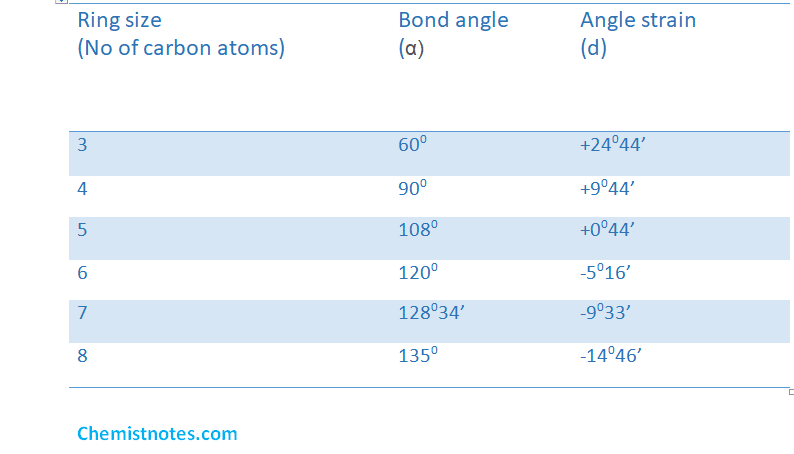
From this table, the following conclusion can be drawn.
- The ‘+’ ve and ‘-’ ve values of ‘d’ indicates that whether the bond angle is less than and greater than the regular tetrahedron bond angle unstable.
- Cyclobutane has less value of angle of strain as compared to cyclopropane.
- Cyclopentane has least value of angle of strain. Hence, it must be stable.
- Cyclohexane has slightly higher strain than cyclopentane.
- Cyclopentane and other cycloalkanes have higher values of angle strain and are highly stable
- At last, rings smaller or greater than cyclopentane or cyclohexane possess higher angle strain and hence these are unstable.
Baeyer’s strain theory limitations
According to Baeyer’s Strain Theory of cycloalkanes, a ring system greater than cyclopentane and cyclohexane should be unstable. But experimentally, it is found that the heat of combustion of cycloheptane is about 158.3 kcal/ mole which is very close to that of cyclopentane or cyclohexane.
Thus, Baeyer’s Strain Theory explains the relative stabilities of ring size up to 5 and 6 in a better way. It can’t explain the exact stability of the higher ring system. This is the failure of Baeyer’s strain theory.
Baeyer’s strain theory video:
References:
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987
- Michael B. Smith, March’s Advanced Organic Chemistry, (7th Edition), John Wiley and Sons, Inc., 2013.
- J. March, Advanced Organic Chemistry, (4th Edition), John Wiley and Sons, 1992






