Table of Contents
ToggleCycloalkanes are a class of hydrocarbons defined by their cyclic structure, wherein the carbon atoms within the molecule are arranged in the shape of a closed ring. Cycloalkanes are saturated hydrocarbons, denoting the absence of double or triple bonds between the carbon atoms constituting the ring structure, thereby exclusively featuring single bonds. Additionally, there exist polycyclic alkanes, which are chemical compounds comprising multiple interconnected cycloalkanes, resulting in the formation of multiple ring structures.
These include both carbon-hydrogen bonds and carbon-to-carbon single bonds, which occur when two carbon atoms combine to form a ring or other cyclic structure. Cyclopropane is often regarded as one of the smallest cycloalkanes.
What is cycloalkane?
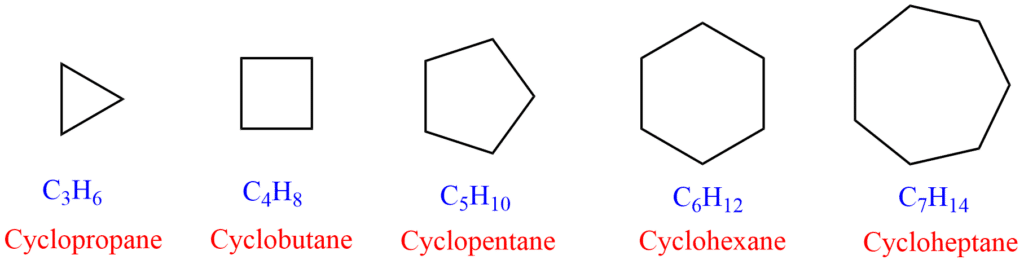
Cycloalkanes are a class of hydrocarbons with carbon atoms connected in a ring structure. The typical formula for cycloalkanes is CnH2n, where n is the number of carbon atoms (3, 4, or 5. and so on) in the molecule.
Examples of cycloalkane
Some examples of cycloalkanes are:
Properties of cycloalkane
The physical properties of cycloalkanes exhibit similarity to those of alkanes with open chains. However, it is observed that cycloalkanes exhibit higher values of boiling point, melting point, and density. This phenomenon can be attributed to the significant presence of London forces, also known as dispersion forces. Cycloalkanes exhibit a higher level of reactivity in comparison to alkanes. The presence of angle strain in ring structures is responsible for this phenomenon.
Some of its main properties are:
- Higher melting and boiling point
- Higher density
- Highly reactive
- Have angle strain
- Insoluble in water
- They possess an extensive amount of London or dispersion forces.
- The carbon atoms in their ring structure are in close proximity.
- At normal temperatures, the first four groups of cycloalkanes are considered to exist in a gaseous state.
- Saturated compounds form a ring structure, hence these are also referred to as saturated hydrocarbons.
- The lack of polarity between the bonds in these compounds is attributed to the low electronegativity between the carbon-hydrogen atoms.
- When compared to other cycloalkanes, cyclopropane is reported to be the most reactive chemical.
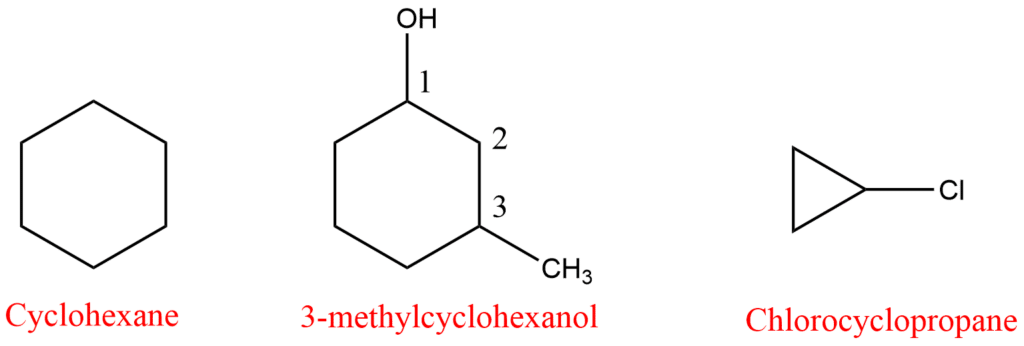
IUPAC Nomenclature of cycloalkane
- The name of alicyclic compounds is derived by adding another prefix ‘cyclo’ followed by the suffix that corresponds to the number of carbon atoms in the ring.
- The suffixes ane, ene, or yne are written depending upon saturation or unsaturation in the ring. Since we are talking about cycloalkane which is a saturated compound, its IUPAC name will be cycloalkane.
- If some substituents or functional group is present, it is indicated by some appropriate prefix or suffix and its position is indicated by numbering the carbon atom of the ring. The numbering is done in such a way as to assign the least possible number to the functional group or substituents. Meanwhile, there is no need to numbering for a single substituent.
- In cases where multiple substituents are present, the substituent group with the highest priority is assigned the lowest numerical value at the corresponding position. When both substituents belong to the same priority group, a system of alphabetical order is employed.
Preparation of cycloalkane
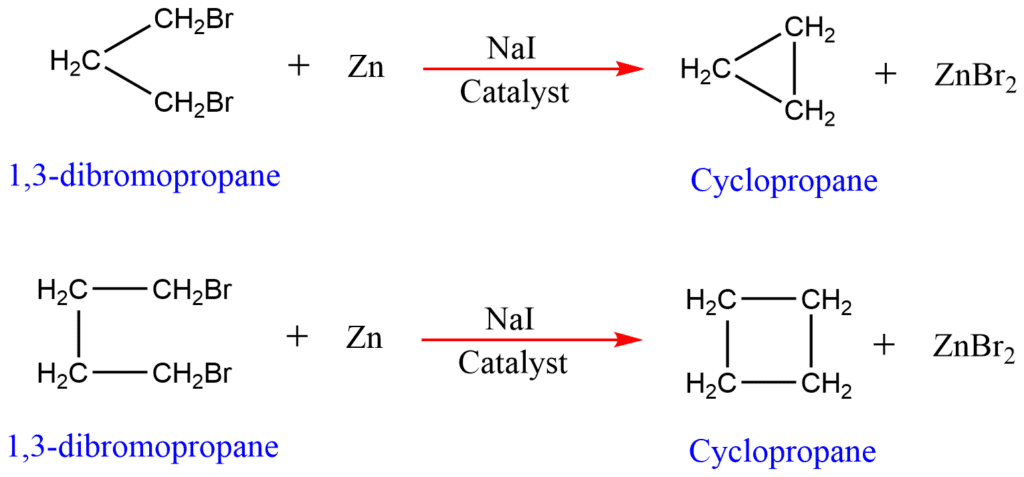
1. From dihalogen derivative of alkane
A dihalogen compound containing halogens on terminal positions, on treatment with Zn, in the presence of NaI catalysts gets dehalogenated. The terminal halogen atoms are removed thereby forming the ring.
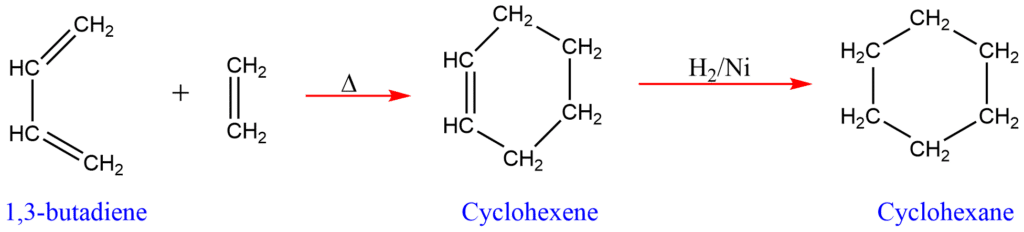
2. Dields-Alder reaction
The (4π + 2π )-Cyclo addition reaction between diene and dienophile in the presence of heat or light to give cyclic compounds is called the Diels-Alder reaction. Two unsaturated compounds can be combined to produce cycloalkane.
3. Reduction of aromatic compounds
Cyclohexane and its derivatives can be prepared by passing hydrogen gas through benzene or its derivatives, in the presence of Ni at 150-200oC.

Uses of cycloalkanes
- In the pharmaceutical industry, they are employed as organic solvents.
- Cancer patients are treated with carboplatin, a drug synthesized from cyclobutane.
- Some types of cycloalkanes are utilized as perfumes in the perfume industry and as pigments.
- Steroids, which are saturated hydrocarbons, can be detected in the tissues of both plants and animals.
- Cyclopropane, a cycloalkane, is employed as an anesthetic agent in the field of medicine.
- It is also utilized in petroleum industries
- These compounds are employed in the production of hair products, and also in the food industries.






