Table of Contents
ToggleThe Sharpless epoxidation deals with the ratio of enantiomers formed in the epoxidation of allyl alcohol (RR’C=CR”CH2OH) with tertiary butyl hydroperoxide and titanium tetra alkoxide in presence of optically active dialkyl tartrate. The Sharpless rule also known as Sharpless epoxidation or Sharpless asymmetric epoxidation involves stereoselective synthesis. Any reaction in which only one stereoisomer is formed predominantly is called a stereoselective synthesis. A stereoselective synthesis may be either enantioselective or diastereoselective. If one of the two enantiomers is formed predominantly, then it is called enantioselective.
While some enantioselective synthesis of epoxide from allyl alcohol using molybdenum and vanadium catalysts is known, Sharpless and Ketsuki (1980) developed the most spectacular stereoselective synthesis employing titanium catalyst epoxidation with tartaric ester as chiral ligand.
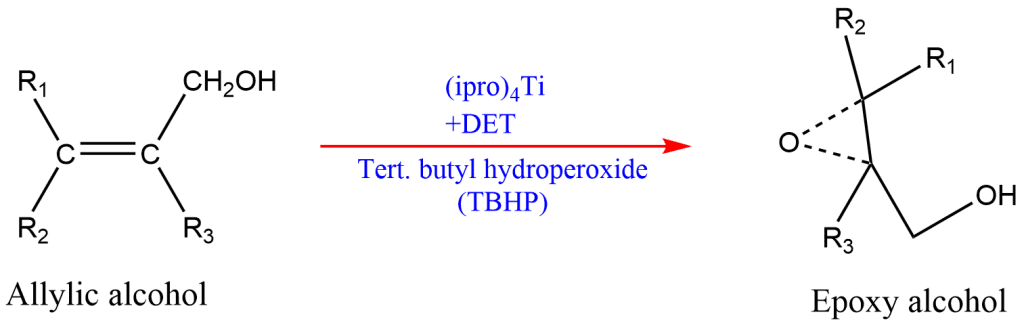
The Sharpless rule/epoxidation is used for the determination of relative configuration by stereoselective synthesis of known stereochemical course.
Sharpless epoxidation mechanism
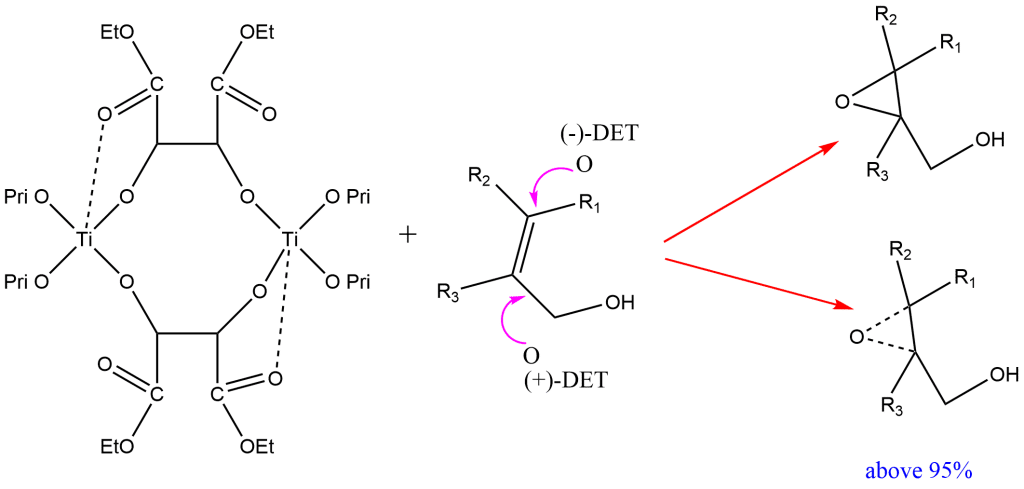
This method consists in treating allyl alcohol with tertiary butyl hydroperoxide in the presence of titanium tetra isopropoxide and optically active (RR or SS) diethyl tartrate (DET). The method relies on a catalyst formed from titanium tetra(isopropoxide) and diethyl tartrate. Here, titanium tetra isopropoxide acts as an oxidizing agent. The ten-membered intermediate catalyst is thought to be formed when titanium alkoxide releases two molecules of isopropyl alcohol. The dominating epoxide in this epoxidation has a predictable absolute configuration.
With (-) Diethyl tartrate (DET), oxygen approaches the double bond from the top while with (+) DET, oxygen approaches the double bond from the bottom giving other isomers. Only the absolute configuration of DET affects the epoxide products’ absolute configuration i.e. the absolute configuration of epoxide depends on the absolute configuration of DET.
The enantioselective is up to 95%.
In 2001, K. Barry Sharpless got a Nobel prize for this and some other work related to asymmetric oxidation.
Application of Sharpless epoxidation reaction
- Determination of relative configuration by stereoselective synthesis of known stereochemical course.
- For the conversion of allylic alcohol into epoxy alcohol
- The total synthesis of many saccharides, leukotrienes, terpenes, pheromones, and antibiotics has been accomplished using Sharpless epoxidation.
Sharpless epoxidation Video
References
- E. L. Eliel, S. H. Wilen and L. N. Mander, Stereochemistry of Organic Compound, John Wiley & Sons, Inc., 1994.
- K. Mislow, Introduction to Stereochemistry, W.A. Benjamin Inc, 1966.
- K. R. Palak, Stereochemistry, Pairavi Prakashan, Kathmandu, Nepal, 2017.






