Table of Contents
ToggleBarton reaction mechanism, examples, and its application in organic chemistry are discussed here:
Barton Reaction
Barton reaction is a photochemical conversion of nitrite ester into δ-nitroso alcohol upon exposure to ultraviolet radiation. The reaction involves homolytic cleavage of RO–NO followed by δ-hydrogen abstraction, and takes place in a liquid phase. This reaction is popularly called as Barton nitrite ester reaction. The geometry of the 6-membered radical intermediate accounts for the selectivity of the δ-hydrogen.
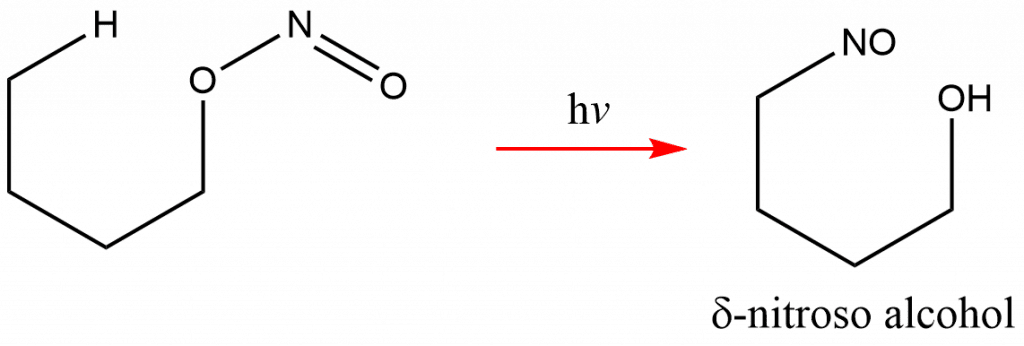
Only starting materials with the proper structure and geometry exhibit the Barton reaction, and photolysis of nitrite esters generally results in unproductive fragmentation, disproportionation, or unselective intermolecular hydrogen abstraction.
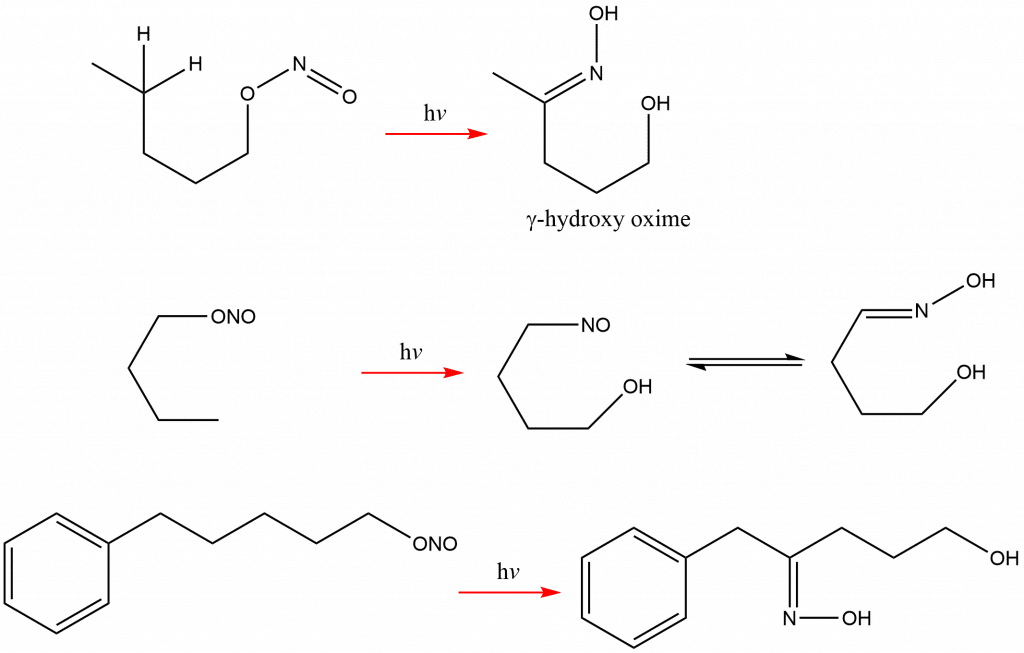
Examples of Barton reaction
Some of the examples of Barton reaction are:
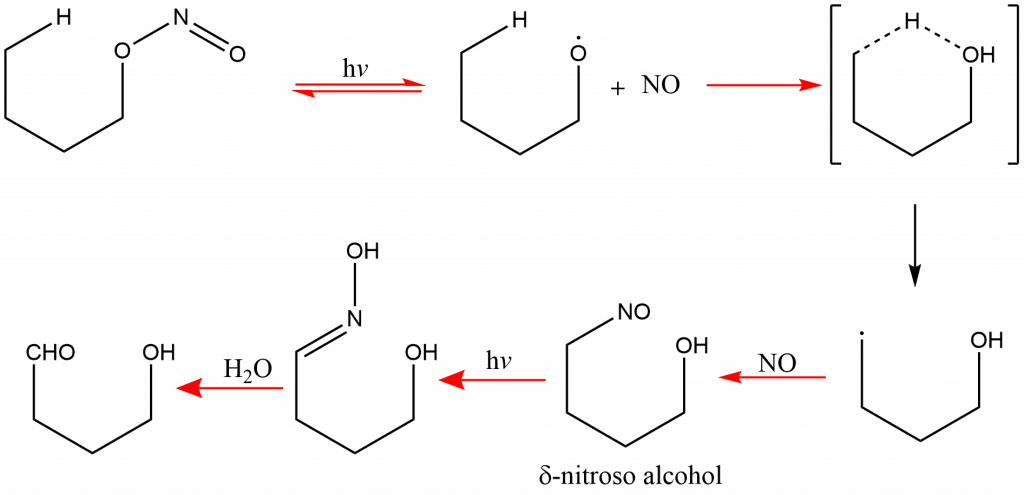
Barton Reaction Mechanism
The nitrous acid ester decomposes upon irradiation, releasing nitrous oxide and alkoxy radical species. The further reaction produces an intermediate carbon radical species, which subsequently undergoes intramolecular hydrogen abstraction via a cyclic six-membered transition state to produce the δ-nitroso alcohol. The formed nitroso molecule can further undergo tautomerization resulting in the formation of oxime derivative, which can then hydrolyze into an aldehyde or be oxidized into nitrile. This mechanism is similar to Hofmann–Löffler reaction.
The Barton reaction mechanism, thus can be ilustarated as:
Applications of Barton Reaction
This reaction is useful in the conversion of the angular methyl group in steroids into the carbonyl group. Some other useful applications are:
- In the preparation of 1-dethia-3-aza-1-carba-2-oxacephem
- Synthesis of a new carbacephem antibiotic, and so on.
Barton Reaction Video
References
- Parikh, A., Parikh, H., & Parikh, K. (2006). Barton Reaction. In Name Reactions in Organic Synthesis (pp. 43-44). Foundation Books. doi:10.1017/UPO9788175968295.013.
- Barton, D. H. R.; Beaton, J. M.; Geller, L. E.; Pechet, M. M. (1960). “A New Photochemical Reaction”. Journal of the American Chemical Society. 82 (10): 2640–2641.






