Table of Contents
TogglePasserini reaction mechanism, examples, and applications in organic chemistry are discussed here:
Passerini reaction
Passerini reaction is a multi-component reaction between a carboxylic acid, a carbonyl compound (aldehyde or ketone), and an isocyanide leading to the formation of an α-acyloxy amide. In other words, it is a three-component condensation reaction of isocyanide, carboxylic acid, and an aldehyde or ketone to form α-acyloxy amide via acyl rearrangement and tautomerization. Passerini reaction is also known as Passerini multicomponent condensation, Passerini condensation, or Passerini three-component coupling.
Passerini reaction can be represented as:
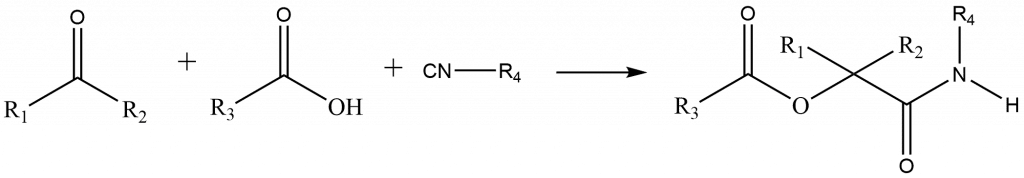
Passerini reaction mechanism
There are two possible mechanisms of passerini reactions i.e. Ionic mechanism and the Concerted mechanism. The reaction takes place by an ionic mechanism in the presence of polar solvents such as methanol or water, while in non-polar solvents, the reaction proceeds via a concerted mechanism.
In the ionic mechanism, Protonation of the carbonyl compound (aldehyde) is followed by the nucleophilic addition of the isocyanide to produce the nitrilium ion. The addition of carboxylate results in intermediate, which on acyl group transfer and amide tautomerization yields the required product, ester.
In a concerted mechanism, A three-component reaction between the isocyanide (R–NC), carboxylic acid, and carbonyl compound (aldehyde) in a series of nucleophilic additions are involved. A 5-membered ring with partial covalent or double bonding is formed as an intermediate and, the whole reaction proceeds via a concerted mechanism in non-polar solvents.
Application of Passerini reaction
This reaction is widely used in combinatorial synthesis. It is usually preferred in the synthesis of natural products, polycyclics, macrocycles, heterocycles, and so on.
Passerini reaction Video
FAQs/MCQs
What is Passerini reaction?
Passerini reaction is such a multi-component reaction of a carboxylic acid, a carbonyl compound (aldehyde or ketone), and an isocyanide that leads to the formation of an α-acyloxy amide.
Is Passerini reaction related to Ugi reaction?
Yes, passerini reaction is closely related to Ugi reaction.
References
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
- Passerini, M., Gazz. Chim. Ital., 1921, 51, 126. (b) Passerini, M., Gazz. Chim. Ital., 1921,51, 181.
- Basso, A.; Banfi, L.; Riva, R.; Piaggio, P. and Guanti, G., Tetrahedron Lett., 2003, 44, 2367.
- Klein, J. C.; Williams, D. R. Passerini reaction. In Name Reactions for Homologations-Part II; Li, J. J., Corey, E. J., Eds.; Wiley & Sons: Hoboken, NJ, 2009, pp765−785. (Review).






