Table of Contents
ToggleConformational isomers of cyclohexane and the stability of these isomers have been discussed in detail along with a suitable diagram. Cyclohexane can adopt different conformational isomers. Let’s discuss them in detail with an energy profile diagram.
Conformational isomers of cyclohexane
Among different conformers of cyclohexane, chair conformation is the most important and stable one. Actually, there are the following different types of conformational isomers of cyclohexane.
- Chair form
- Half-chair/half boat form
- Twisted boat form
- Boat form
Two Chair conformation rapidly interchange via passing through different conformation having high energy, such as half chair, twisted boat, and boat conformation. The inversion process can be illustrated as given below:
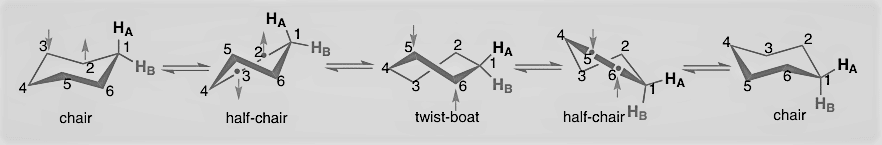
A. Chair form:
In chair form, all C-H bonds are perfectly staggered. This can be explained by drawing Newmann projection.
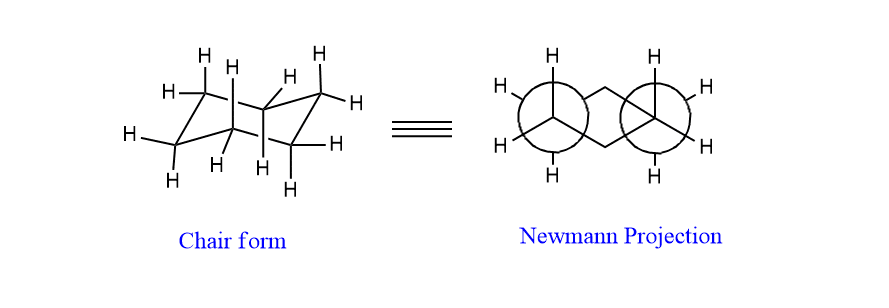
Hence, chair conformation is free from angle strain as well as torsional strain.
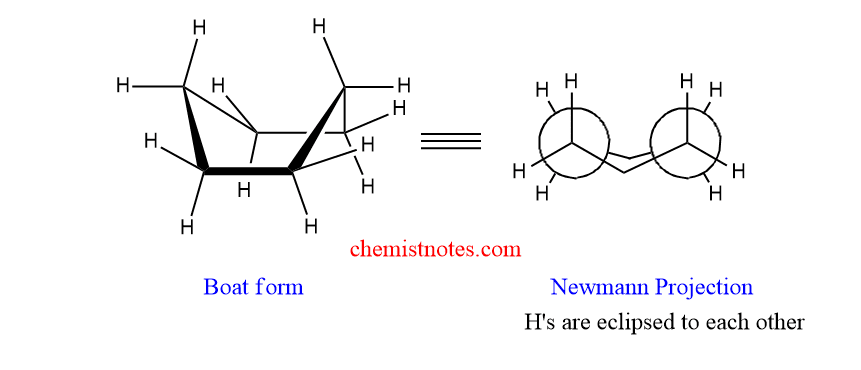
B. Boat form:
In boat from, four C-H Bonds occupy eclipsed position, and the rest of C-H bonds are staggered. It has torsional strain due to eclipsing of C2-C3 and C5-C6 carbon-hydrogen bonds. Therefore, the boat form is less stable than the chair form.
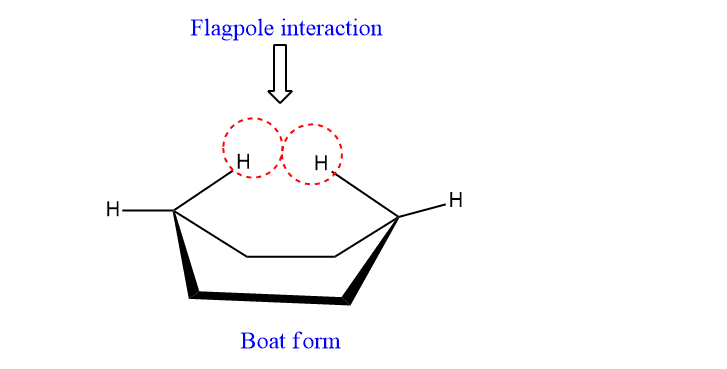
The boat form has most of H’s eclipsed and other H’s of the ring interact, increasing the energy of the system. Such interaction of H’s is called flagpole interaction.
C. Twisted-boat form:
When the boat conformation of cyclohexane is twisted in such a way that C2 and C5 come up and C3 and C6 go down, a new conformation is obtained called twisted-boat form. The boat form tends to decrease some of its torsional strain by twisting and giving new conformation known as twisted boat form.
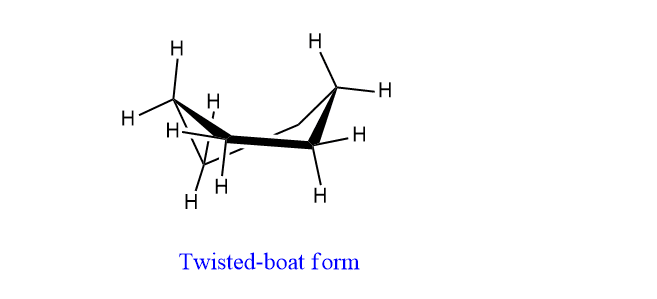
This twisted boat form of cyclohexane can undergo changes into another enantiomer, twisted boat form by climbing an energy barrier of 7 kJ/mole at the top of which is the boat conformation.
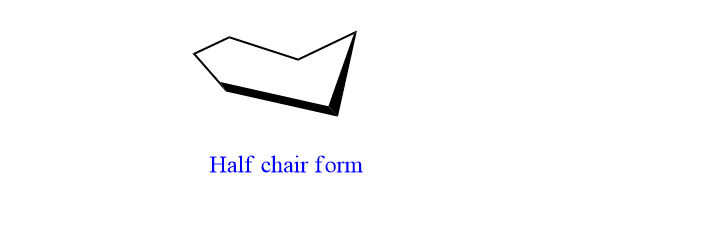
D. Half Chair form:
Chai form undergoes twisting in such a way that one end remains the same and the other ends lie horizontally. This form has very high energy and it is highly unstable and considered as transition conformational isomer of cyclohexane.
Stability of conformational isomers of cyclohexane
The energy profile diagram representing the different conformational isomers of cyclohexane is shown below:
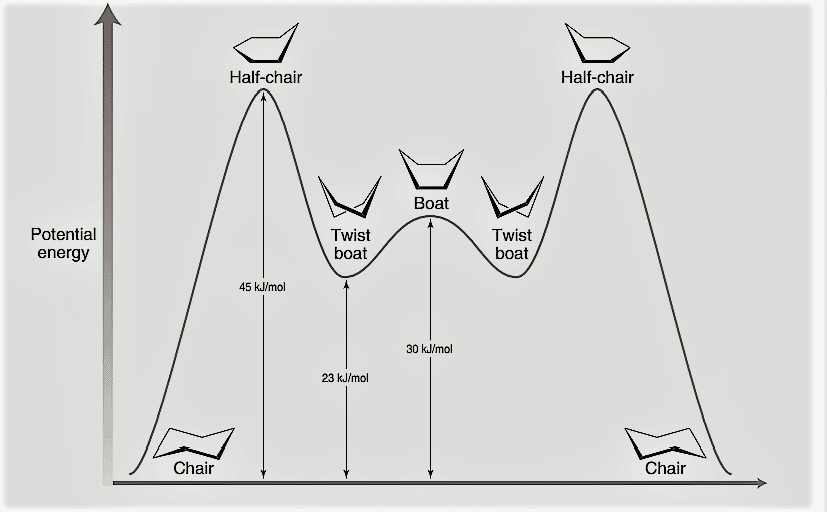
- The twisted boat form of cyclohexane is less stable than chair conformation by about 23 kJ/mole.
- The twisted boat form is more stable than the boat conformation by about 7 kJ/mole.
- The energy barrier separating the chair form (I) and one of the twisted boat form(III) corresponds to the transition state conformation(II), which is called as the half chair or half boat form of cyclohexane.
- The half boat has 45 kJ/mole more energy than chair conformation. Such high energy of the half boat conformation is due to both angle strain and torsinal strain.
- Therefore, the chair form (I) and two twisted boat forms(III and V) are conformers while the half chair form and the boat forms are considered as transition state conformations of cyclohexane.
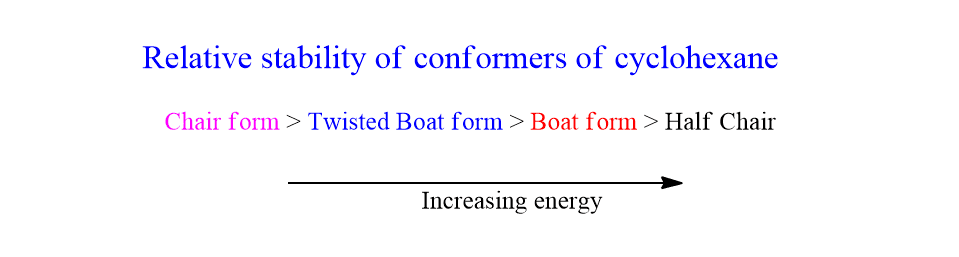
The relative stability order of conformational isomers of cyclohexane is given as:
References
- J. Clayden, N. Greeves, and S. Warren, Organic Chemistry, (2nd Edition), Oxford
University Press, India, 2014. - Klein, David R. Organic Chemistry as a Second Language: Second Semester Topics. 3rd ed. Wiley, 2012.






