Table of Contents
ToggleOppenauer oxidation, examples, mechanism, and application have been discussed here.
oppenauer oxidation definition
The aluminum tri-isopropoxide catalyzed oxidation of secondary alcohol into ketones using another ketone or an aldehyde as the oxidant is called oppenauer oxidation reaction. Oppenauer oxidation is the reverse process of Meerwein-Ponndorf-Verley Reduction.
Note: Oppenauer oxidation reaction does not affect carbon-carbon double bond, triple bond, amino group, aldehyde group, halogens, and sulfur-containing functional group. Therefore, this reaction shows high selectivity. This method is a very common method for the preparation of ketones.
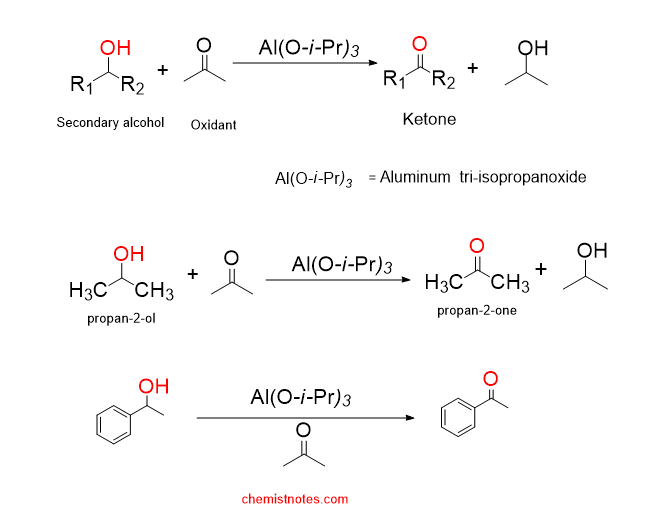
oppenauer oxidation reaction mechanism
Let’s see how the oppenauer oxidation reaction proceeds. The mechanism of the oppenauer oxidation reaction is illustrated as shown below:
Let’s illustrate it stepwise
Step 1: The lone pair of the oxygen atom of alcohol attacks the aluminum center of the aluminum tri-isopropoxide.
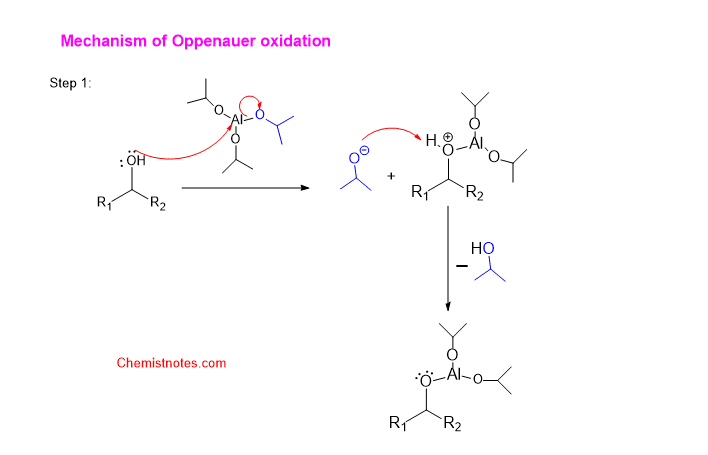
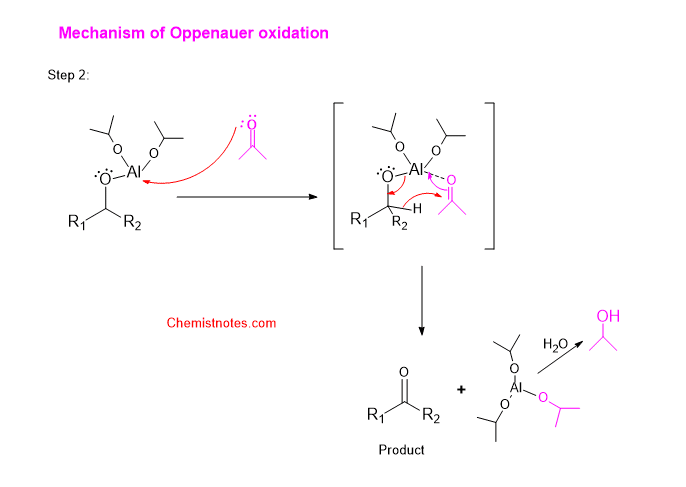
Step 2: The other ketone as an oxidant and above-formed adduct react to form a cyclic transition state, which breaks down to give a product.
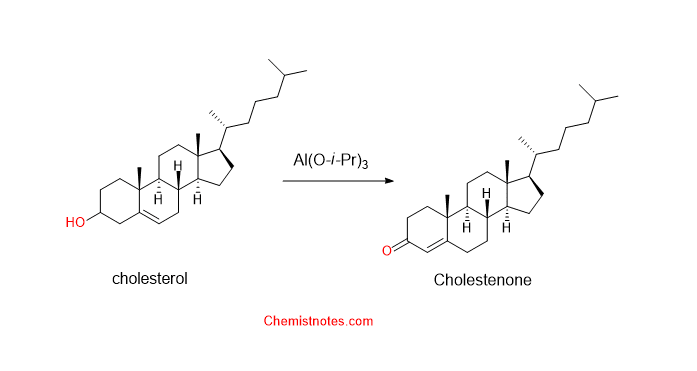
oppenauer oxidation of cholesterol
Cholesterol on oppenauer oxidation, the OH group is converted into a carbonyl group. But the C=C double bond is not affected. The oppenauer oxidation of cholesterol is given by the following reaction:
oppenauer oxidation applications
The application of oppenauer oxidation reaction is to convert the secondary alcohol to ketones. Since this reaction is highly selective, it is useful in the preparation of ketones from alcohol.
- It has been reported that oppenauer oxidation is used in the preparation of morphine in pharmaceutical industries.
- It has been also reported that progesterone is prepared by the oppenauer oxidation of pregnenolone.
Oppenauer oxidation Video
References:
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
If you want to learn about Baeyer villiger oxidation reaction, then click here.
FAQs/MCQs:
What is oppenauer oxidation?
Oppenauer oxidation is a type of oxidation reaction in which secondary alcohol is converted in to ketones using aluminum tri-isopropanoxide catalyst.






