Table of Contents
ToggleChromic acid oxidation, examples, mechanism, and some applications have been discussed here.
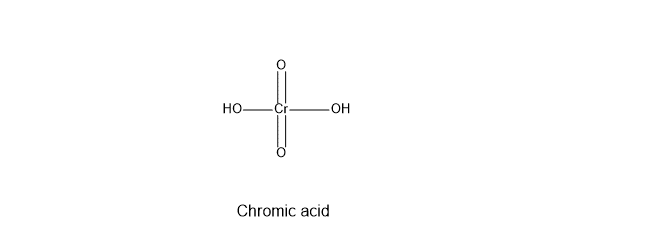
Chromic acid oxidation is used for the oxidation of alcohols, aldehydes, and carbon-carbon double bonds in the hydrocarbons. Chromic acid has the formula H2CrO4 and has a structure as shown below. The chromic acid is generated during the reaction by mixing the following reagents:
- K2Cr2O7 and H2SO4
- Na2Cr2O7 and H2SO4
- K2CrO4 and H2SO4
- CrO3 and H2O
Don’t get confused if we use these mixtures instead of Chromic acid H2CrO4.
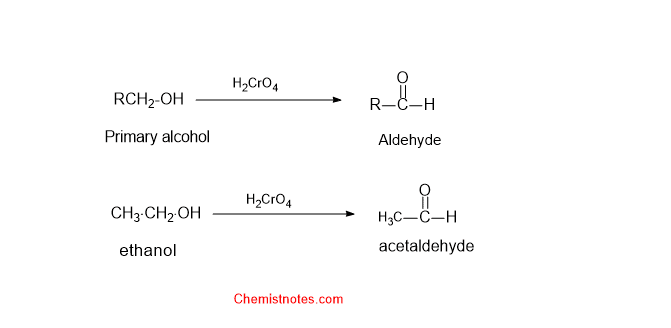
Oxidation of alcohol by chromic acid
Chromic acid can oxidize primary and secondary alcohol into aldehyde and ketones respectively. But it can not oxidize tertiary alcohols. We will know why tertiary alcohols do not undergo oxidation later by observing the mechanism of the reaction.
Oxidation of primary alcohol:
When primary alcohol is oxidized by chromic acid, primary alcohol is converted into an aldehyde. Let’s see some examples:
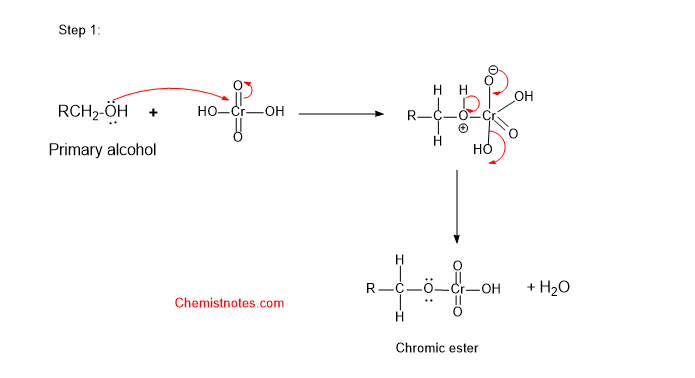
Primary alcohol oxidation mechanism by chromic acid
Let’s see how the oxidation of primary alcohol by chromic acid takes place. The mechanism completes in the following steps.
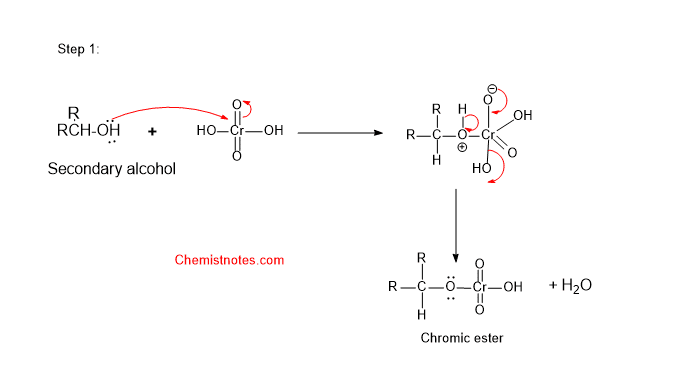
Step 1: First of all, alcohol reacts with chromic acid (H2CrO4) to form an ester of chromic acid. The oxygen atom of alcohol attacks the chromium atom of the chromic acid.
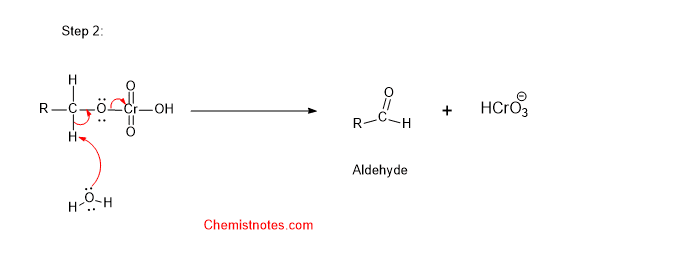
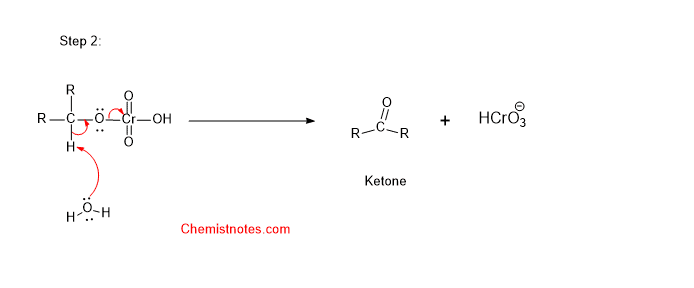
Step 2: In the second step, water molecule abstracts a hydrogen atom from the alcohol.
Oxidation of Secondary alcohol:
When secondary alcohol is oxidized by chromic acid then secondary alcohol is converted into ketones. Let’s see examples:
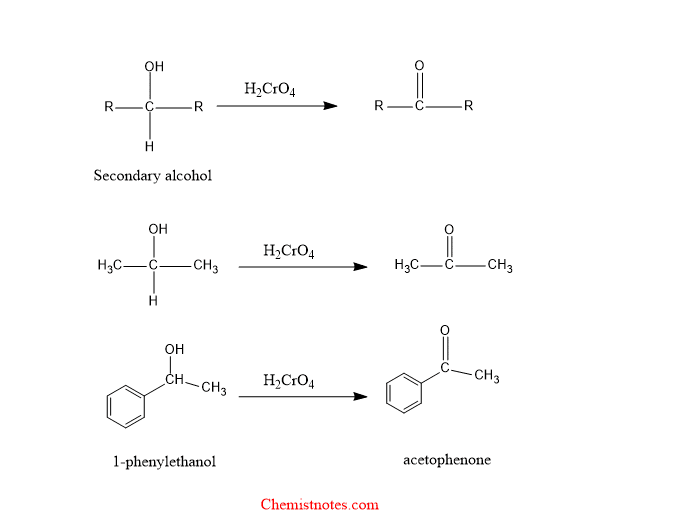
Secondary alcohol oxidation mechanism by chromic acid
The secondary alcohol is oxidized in a similar manner as primary alcohol. The mechanism is also similar. The mechanism completes in following steps:
Step1 :
Step 2:
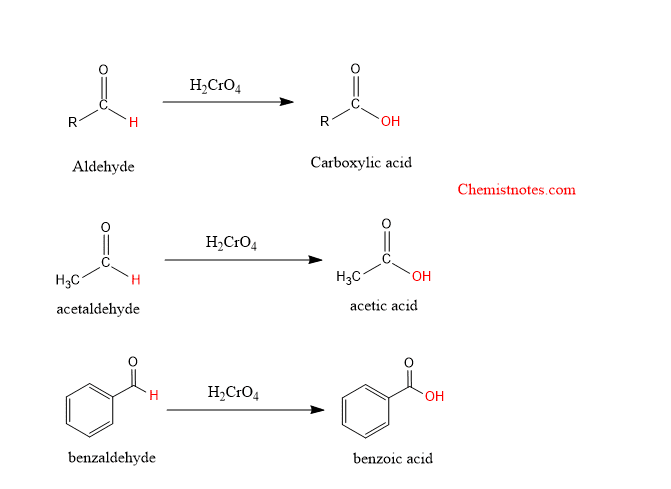
Oxidation of aldehyde with chromic acid
The aldehyde can be further oxidized with chromic acid to carboxylic acid. Let’s see examples:
Aldehyde oxidation mechanism:
Let’s see the mechanism of the oxidation of aldehyde. The overall mechanism completes in the following steps:
Step1 : The lone pair of electrons of oxygen in the water molecule attacks the carbonyl carbon.
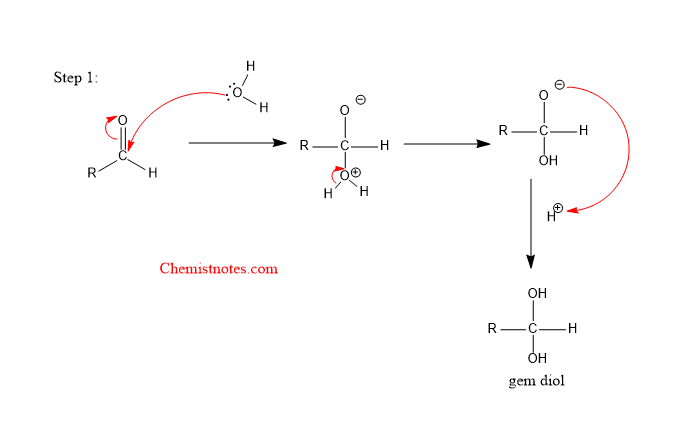
Step 2: The lone pair of electrons of oxygen of gem diol attack the chromium atom of the chromic acid.
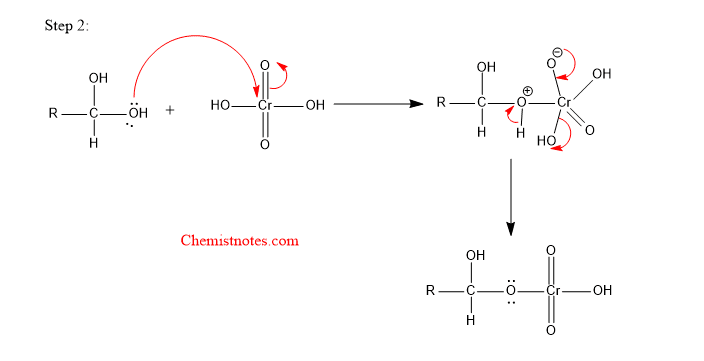
Step 3:
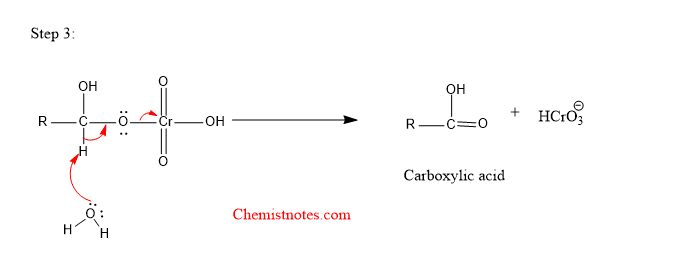
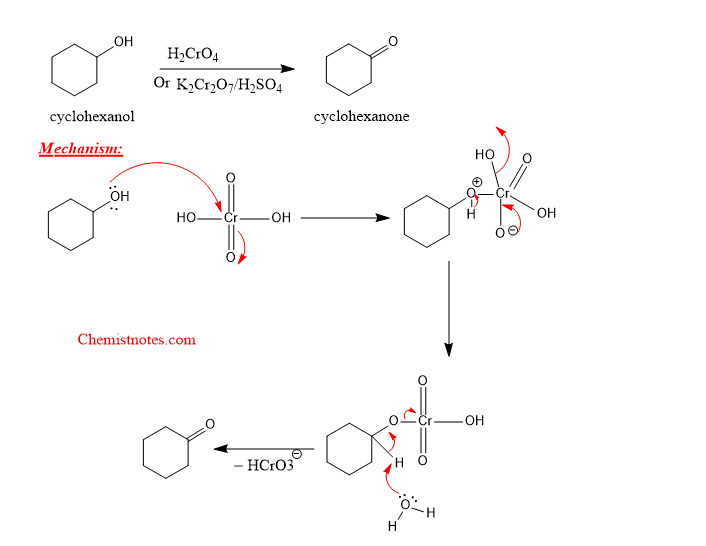
Oxidation of cyclohexanol with chromic acid
When cyclohexanol is treated with chromic acid, cyclohexanol is oxidized to cyclohexanone as shown below:
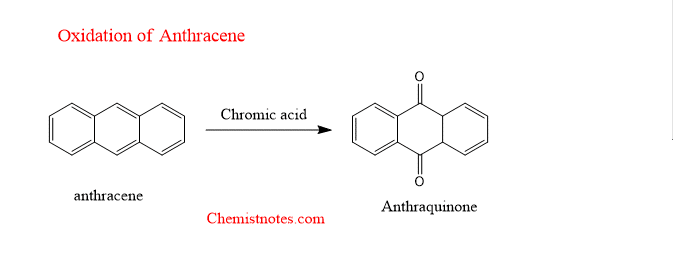
oxidation of anthracene with chromic acid
Anthracene undergoes chromic acid oxidation to form anthraquinone.
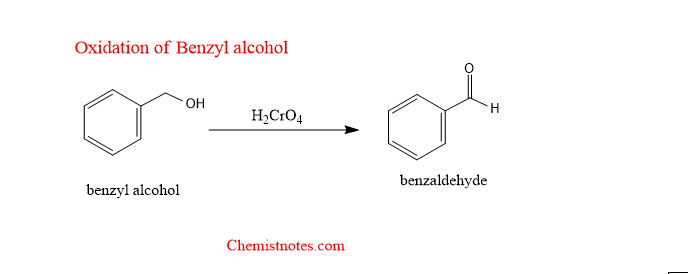
oxidation of benzyl alcohol with chromic acid
Benzyl alcohol is oxidized with chromic acid to benzaldehyde.
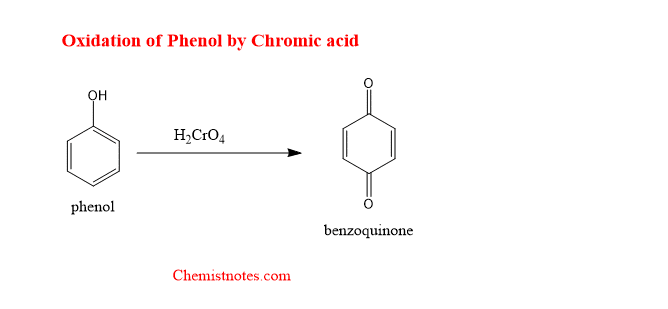
Oxidation of phenol with chromic acid
When phenol is oxidized with chromic acid, the phenol is converted into benzoquinone.
Chromic acid oxidation video
References:
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
If you want to know more about oxidation reactions such as oppenauer oxidation, then click here.
Please comment down if you have any problems related to this topic. Thank you.
FAQs/MCQs:
Does chromic acid oxidize tertiary alcohol?
No, tertiary alcohol can not be oxidized by chromic acid under mild conditions.






