Table of Contents
ToggleBaeyer villiger oxidation reaction, examples, mechanism, and its applications have been discussed here.
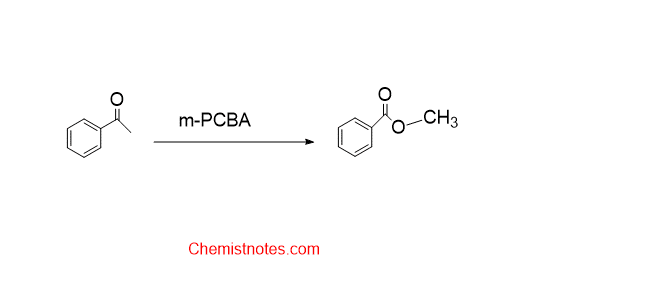
Baeyer villiger oxidation reaction
The oxidation of ketones or aldehyde to ester in the presence of hydrogen peroxide or organic peroxides such as peroxy acids, perbenzoic acid, or peracetic acid is called the Baeyer villiger oxidation reaction. An acidic catalyst is required for this reaction.
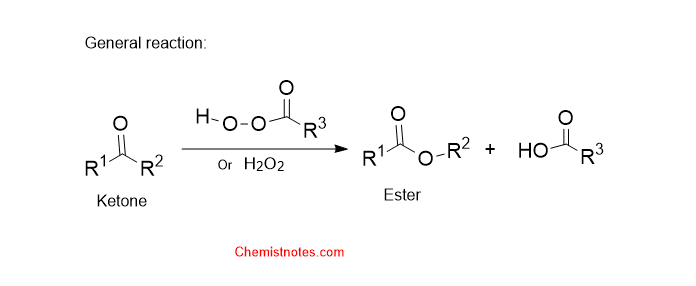
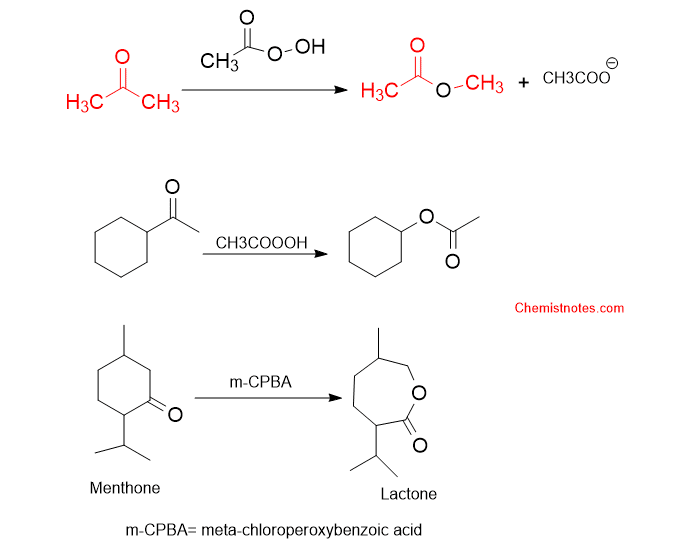
Let’s see some specific examples of Baeyer villiger oxidation.
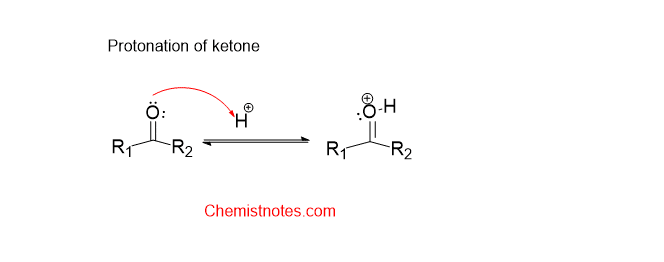
Baeyer villiger oxidation mechanism
The mechanism of the Baeyer villiger oxidation is given below:
Step1: Protonation of the carbonyl group
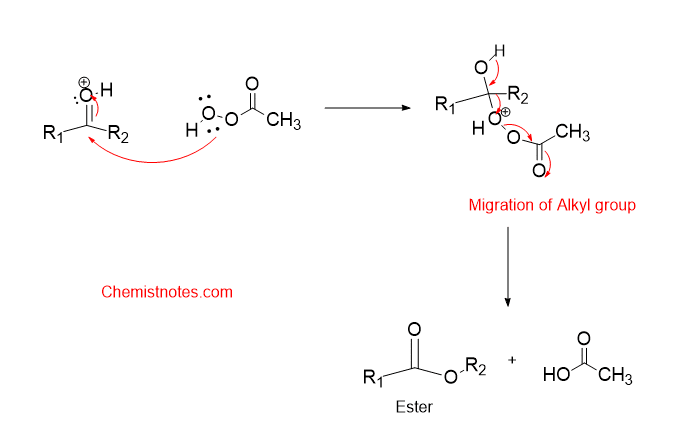
Step 2: The one pair present on the oxygen atom of the peroxy acid attacks the carbonyl carbon followed by the migration of the alkyl group. Note that only the more substituted carbon migrates first i.e most electron alky group.
Baeyer villiger oxidation application
The main application of the Baeyer villiger oxidation reaction is the preparation of ester or carboxylic acid from ketones or aldehyde.
References:
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987
- J.J. Li, Name Reactions, 4th ed.© Springer-Verlag Berlin Heidelberg 2009.
If you want to know about Hydroboration oxidation reaction, click here.
Please comment down if you have any problems related to this topic. Thank you.






