Table of Contents
ToggleCannizzaro reaction, examples, mechanism, and its applications have been discussed here.
Cannizaro reaction definition
The aldehydic compounds having no α-hydrogen atoms in presence of strong base undergo disproportionation to form an equal amount of corresponding alcohol and carboxylic acids. Such a reaction is called the Cannizzaro reaction. Some of the examples of the Cannizzaro reaction involve the reduction of benzaldehyde, formaldehyde, trimethyl acetaldehyde, etc.
Let’s see the reaction of Cannizzaro reaction.
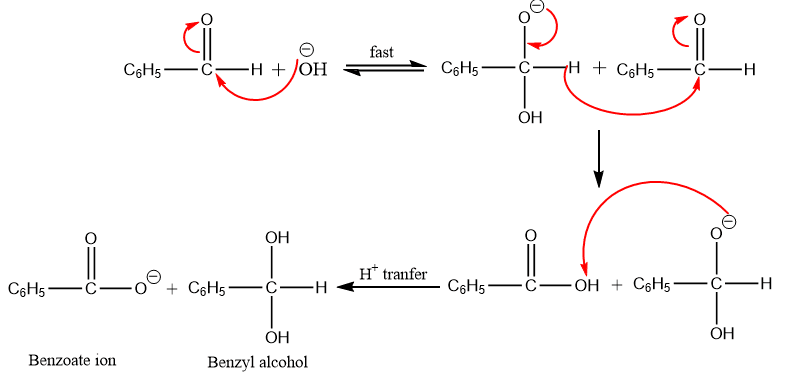
Cannizzaro reaction mechanism
The reaction mechanism is performed by the nucleophilic attack and the transfer of hydride ions from one molecule to another. Here, the reaction involves the reduction of one molecule to alcohol and oxidation of another molecule to carboxylic acid. In other words, one molecule of the aldehyde acts as a hydride (H–) donor, and the other acts as a hydride acceptor.
The mechanism can be described into 3 following steps:
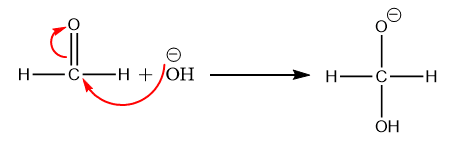
Step 1: Formation of the intermediate by the nucleophilic attack of hydroxide ion to the carbonyl group.
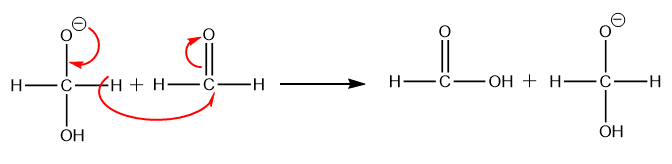
Step 2: Step 2: The hydride ion is transferred directly from the intermediate generated in step 1 to the electron-deficient carbonyl carbon atom of another aldehyde molecule.
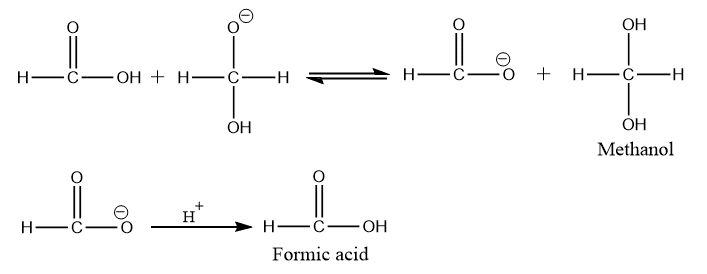
Step 3: Proton exchange occurs quickly between the carboxylic acid and the alkoxide ion (intermediate formed in step 2).
Cannizzaro reaction mechanism of benzaldehyde
The Cannizzaro reaction mechanism of benzaldehyde can be carried out in a similar fashion as that for formaldehyde (described above).
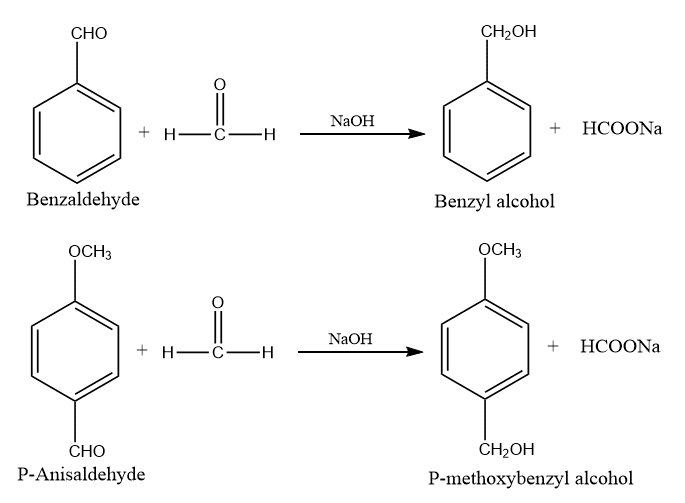
Cross Cannizzaro reaction
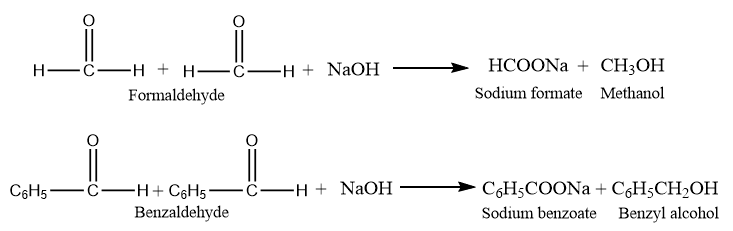
When a mixture of formaldehyde and benzaldehyde is treated with caustic soda, benzyl alcohol along with the sodium formate is obtained. Such a reaction is called the Cross Cannizzaro reaction. It results when an oxidant aldehyde differs from the reductant aldehyde in a Cannizzaro reaction. In this reaction, formaldehyde is oxidized to formic acid, while benzaldehyde is reduced to the equivalent alcohol.
Aldol condensation and Cannizzaro reaction
The aldol condensation reaction is a base or acid-catalyzed condensation reaction between two molecules of carbonyl compounds that are either identical or different, and at least one of them contains α -hydrogen atoms, which results in β -hydroxy carbonyl compounds or, α, β-unsaturated carbonyl compounds. It involves a coupling reaction. An enol or an enolate and a carbonyl compound are the two reactants of this reaction. It entails an easy formation of carbon-carbon bond.
Cannizzaro reaction involves the disproportionation reaction of aldehydic compound having no α -hydrogen atoms in presence of strong base to form corresponding alcohol and carboxylic acid in an equal amount. It involves a redox reaction in which one molecule gets oxidized, and the other gets reduced. Two non-enolizable aldehydes are used as reactants in this reaction. Such reaction proceeds by the transfer of hydride from one molecule to the other to form an acid and alcohol.
Application of Cannizzaro reaction
- Synthesis of mandelic acid
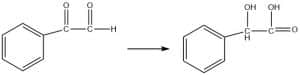
- Preparation of O-carboxybenzyl alcohol by internal Cannizzaro’s reaction

- Polyols are synthesized in the industry using a combination of the crossed Cannizzaro reaction and aldol condensation.
- As Cannizzaro reaction can also take place between different aldehydes, all the possible products are obtained.
Cannizzaro reaction video
FAQs/MCQs
Cannizzaro reaction definition
The reaction in which aldehydic compounds having no α-hydrogen atoms is treated in presence of strong base to form an equal amount of corresponding alcohol and carboxylic acids called as Cannizzaro reaction
Cannizzaro reaction example
Aldehydes having no α-hydrogen atoms like benzaldehyde, formaldehyde, trimethyl acetaldehyde, etc. undergo Cannizzaro reaction.
Cannizzaro reaction between formaldehyde and benzaldehyde
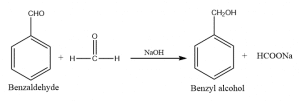
The Cannizzaro reaction between formaldehyde and benzaldehyde results in the formation of benzyl alcohol and sodium formate.
Cross Cannizzaro reaction is given by
The cross Cannizzaro reaction is shown by a mixture of formaldehyde and benzaldehyde when treated with Conc. NaOH.
Cannizzaro reaction mechanism formaldehyde
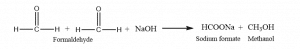
The reaction mechanism involves nucleophilic attack and the transfer of hydride ions from one molecule to another.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.






