Table of Contents
ToggleMethanol, also known as methyl alcohol, is a colorless watery organic liquid and the simplest aliphatic alcohol, with the chemical formula CH3OH. Although it is a toxic alcohol, it finds extensive industrial use industrially as a solvent, pesticide, and even as an alternative fuel source. It occurs naturally in the form of esters and is also called wood alcohol. For example, the oil of wintergreen is known to contain methyl salicylate. Since it was formerly prepared chiefly from wood by a process known as destructive distillation, it is also called wood sprit or wood naphtha.
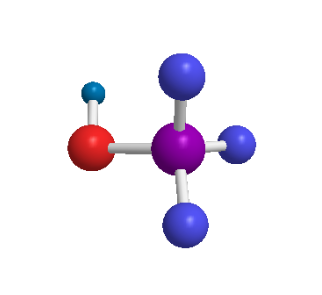
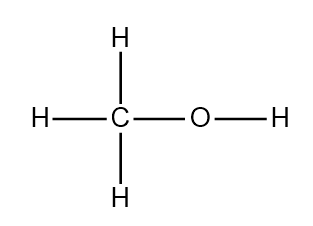
Structure of methanol
Properties of Methanol
- Methyl alcohol is a colorless mobile liquid having a specific gravity of 0.793 and a boiling point of 65oC.
- It has a sharp wine-like odor and a burning taste.
- It is miscible with water in all proportions, the mixing being attended by a contraction in volume.
- It is an excellent solvent for several organic substances such as oils, fats, etc.
- Methyl alcohol is poisonous and when taken internally it causes blindness or even death.
- It gives most of the general reactions of alcohol.
| Chemical Formula | CH3OH |
| Solubility | miscible with most organic solvnets |
| Boiling point | 148.5oF |
| Melting point | -144.5oF |
| Ionization potential | 10.84eV |
| Molecular mass | 32.04 |
| Flammability | Highly flammable |
| Specific gravity | 0.79 |
Preparation of Methanol
Methyl alcohol can be prepared by any of the general methods of preparation of alcohol. On a commercial scale, it is manufactured from water gas which is a mixture of carbon monoxide and hydrogen in a ratio of 1:1. To manufacture methanol, water gas is mixed with half of its volume of hydrogen and is compressed to 200 atmospheric pressure. The compressed gaseous mixture is then passed over the ZnO-Cr2O3 catalyst at 300oC.
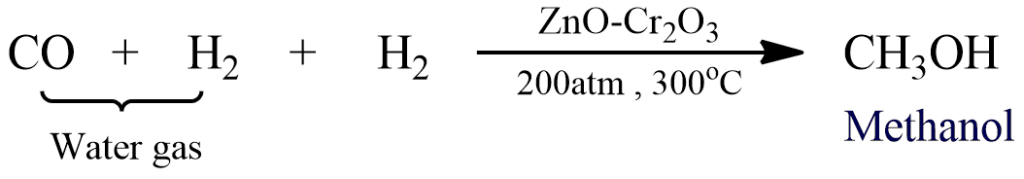
Enzymes, such as methane monooxygenases, influence the catalytic conversion of methane to methanol. These enzymes are mixed-function oxygenases, i.e. oxygenation is coupled with the production of water.

Uses of Methyl Alcohol
- Methyl alcohol is used as a solvent for nitrocellulose, resins, dyes, plastics, paints, varnishes, etc.
- It is also used as an antifreeze for automobile radiators.
- It is used for the denaturation of ethanol.
- It is used as a motor fuel.
- It is used as a dehydrator for natural gas.
- It can also be used in the manufacture of perfumes, drugs, formaldehyde, and dimethyl terephthalate.
How to test methyl alcohol/methanol in the lab?
- About 1 ml of methyl alcohol is taken in a test tube and, a pinch of salicylic acid and a few drops of conc. H2SO4 is added. The content is warmed in a water bath for a few minutes. Methyl salicylate is produced which is recognized by its characteristics of fragrant smell.
- A few drops of methyl alcohol are taken in a wide-mouth test tube. A piece of copper gauze is heated to redness and dropped into the test tube. Methyl alcohol is oxidized to formaldehyde which is at once recognized by its characteristic pungent odor.
- A small quantity of methyl alcohol is added to some water contained in a test tube. A spiral copper wire is heated to redness in the flame of the bunsen burner and is plunged into the solution. The process is repeated several times and the solution is cooled. Now, a few drops of a dilute solution of resorcinol are added and conc. H2SO4 is poured along the side of the inclined test tube so as to form a lower layer. At the junction of the liquids, a pink ring appears with a thick white precipitate above it.
FAQs
What is methanol?
Methanol, also known as methyl alcohol, is a colorless watery organic liquid and the simplest aliphatic alcohol, with the chemical formula CH3OH.
Is methanol polar or nonpolar?
Methanol is a polar solvent.
Is methanol flammable?
Methanol is a highly flammable liquid.
What is methanol used for?
Methanol is extremely used in industry as a solvent, pesticide, and even as an alternative fuel source.
Is methanol soluble in water?
Methanol has good solubility in water.






