Table of Contents
ToggleGenerally, there are six types of Enzymes, and are named by the Enzyme commission rule(EC). IUPAC as we already knew about it, gives the naming of chemical compounds likewise EC is also a naming system having four digits after enzymes names. Based on the chemical reactions that enzymes catalyze, the Enzyme Commission number (EC number) is a system of numerical classification for enzymes. Every EC number in the system of enzyme nomenclature has a suggested name for the corresponding enzyme-catalyzed reaction. They are:
- hydrolases
- oxidoreductase
- lyases
- transferases
- ligases
- isomerases
Enzymes are the biocatalyst that alters the life reaction. All the enzymes are proteins, though some special RNA species also act as enzymes and are called ribozymes example hammerhead ribozymes. The active site of enzymes is the region that binds the substrate, co-factors, and prosthetic group all containing residue that helps to hold the substrate. The general characteristics of enzymes are:
- Enzymes speed up the reaction by decreasing the activation path.
- They are specific in nature.
- The presence of enzymes doesn’t affect the nature and properties of the product.
- Enzymes are sensitive to Ph, temperature, and concentration.
- Enzymes are colloidal in nature and thus, the addition of electrolytes coagulates the enzymes.
Nomenclature of Enzymes:
An enzyme is named according to the name of the substrate it catalyzes. For example, the formal systematic name of the enzyme catalyzing the following reaction is ATP glucophosphotransferase.
ATP + D-glucose = ADP + D- glucose-6-phosphate
It indicates that the enzymes catalyze the transfer of a phosphoryl group from ATP to glucose.
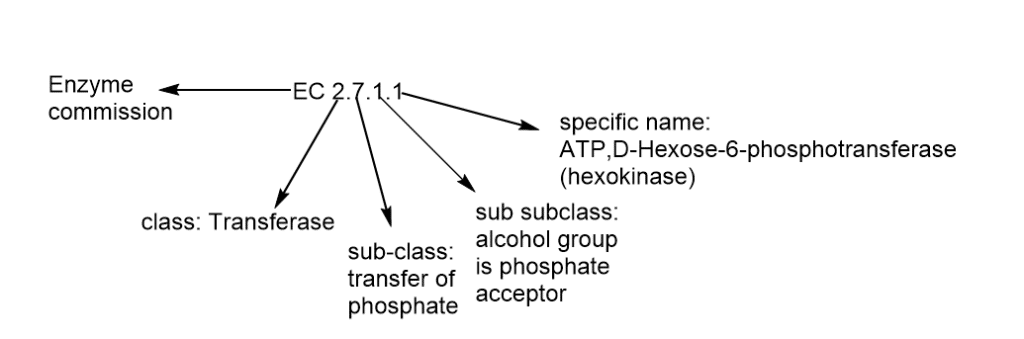
Internationally, each enzyme is assigned a four-part of classification number which helps to identify the reaction it catalyzes. For example
Hexokinase has enzyme commission number E.C (2.7.1.1) these components indicate the following groups of enzymes.
- The first number (2) indicates class transferase.
- The second number (7) indicates subclass phosphotransferase .
- The third number (1) indicates that the sub-sub class phosphate acceptor is (the hydroxyl group).
- The last one (1) indicates (D- glucose as the phosphoryl group acceptor).
Types of Enzymes:
1. Oxidoreductases (E.C.1)
The transfer of electrons from one molecule (the oxidant, hydrogen, or electron donor) to another is catalyzed by oxidoreductases (oxidases, oxygenases, peroxidases) (the reductant, the hydrogen or electron acceptor). These enzymes bring about oxidation and reduction.
Most often, this category of enzymes uses NADP+ or NAD+ as cofactors. Respiratory complexes I, II and III are produced by transmembrane oxidoreductases in mitochondria, chloroplasts, and bacteria. Others can bind to biological membranes via a single transmembrane helix or associate with them as peripheral membrane proteins.

2. Transferases (E.C.2)
These are responsible for transferring functional groups from one molecule to another.
Transferase transfer functional groups example methyl or phosphate between acceptor and donor molecules. Any member of the class of enzymes known as transferases catalyzes the transfer of particular functional groups, such as a methyl or glycosyl group, from one molecule (referred to as the donor) to another (called the acceptor). They are essential to some of life’s most crucial functions and participate in hundreds of various metabolic pathways throughout biology.

3. Hydrolases (E.C.3)
The group of enzymes known as hydrolases is responsible for bond cleavages caused by reactions with water. Most hydrolases have a digestive nature, breaking down nutrients into more manageable portions for digestion. It catalyzes the hydrolysis of various bonds and adds water molecules across a bond.
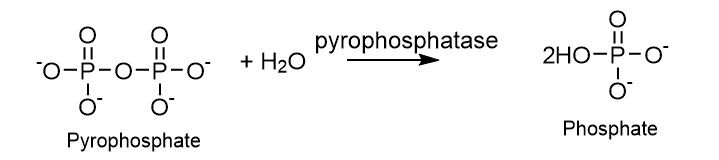
4. Lyases (E.C.4)
In addition to hydrolysis and oxidation, lyases can form or remove double bonds by adding or subtracting water, ammonia, or carbon dioxide. Some examples of these lyases include fumarase, histidase, carbonic anhydrase, etc.
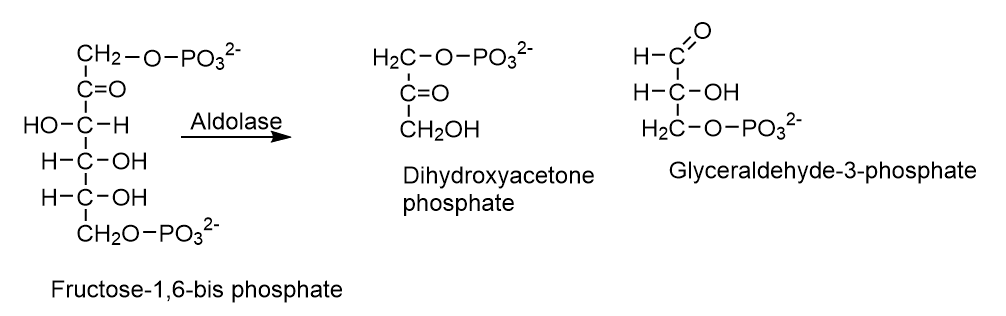
5. Isomerases (E.C.5)
Isomerases catalyze changes in a molecule’s isomer structure. It contains a variety of isomerization reactions, such as l-to-d isomerization and mutase reactions, which involve shifting chemical groups.
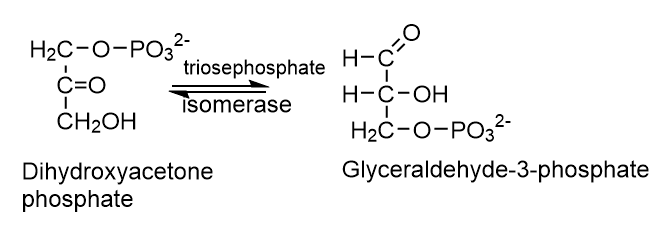
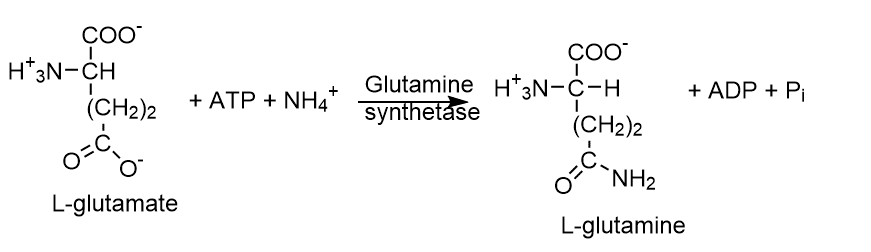
6. Ligases (E.C.6)
Utilizing ATP energy, ligases catalyze synthetic reactions that link two molecules via covalent bonds. Glutamine synthetase, for instance, Acetyl CoA, Carboxylase, etc.
MCQs/FAQs
What are enzymes?
Enzymes are biocatalyst that alters the reaction of life.
How many types of enzymes are there?
There are generally 6 types of enzymes.
How do enzymes function?
The vital function of enzymes is to reduce the activation energy of a reaction or the amount of energy required for the reaction to start. In order to facilitate the chemical bond-forming and bond-breaking processes, enzymes bind to reactant molecules and hold them in place.
Is enzymes a protein?
Enzymes are polypeptide chains made up of amino acids that have been joined to form proteins. A polypeptide chain’s fundamental structure is defined as this arrangement of amino acids.