Table of Contents
ToggleEthyl malonate, CH2(COOC2H5)2, is commonly called a malonic ester. Malonic ester synthesis refers to the synthesis of a wide range of organic compounds starting with malonic ester. The synthesis is based on the following two facts:
- The higher acidity of ∝-hydrogens of malonic ester and
- The extreme ease with which malonic acid and substituted malonic acids decarboxylated.
Mechanism of Malonic Ester Synthesis
The synthesis involves the following steps:
- The ∝-hydrogens in malonic ester are fairly acidic. When malonic ester is treated with a strong base such as sodium ethoxide in absolute alcohol, it is covered into its salts, known as sodiomalonic ester.

- The enolate ion is a nucleophile and therefore this step involves nucleophilic attack on the alkyl halide by the carbanion,
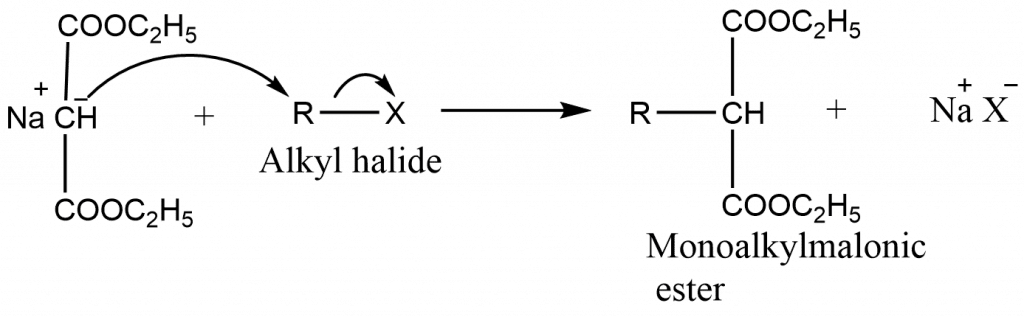
The monoalkylmalonic ester still has one acidic hydrogen. Therefore, it can be alkylated again using same or different alkylhalides to get dialkylmalonic ester.
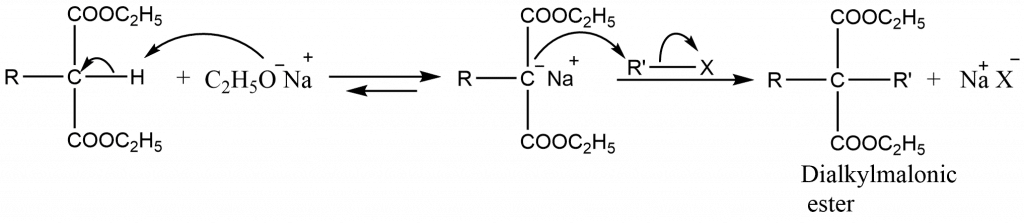
- The mono- or dialkylmalonic ester thus obtained can be hydrolysed to give a mono- or dialkylmalonic acid. These acids undergo decarboxylation readily to form mono- or disubstituted acetic acid.
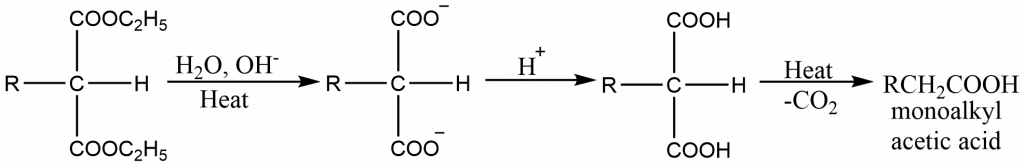
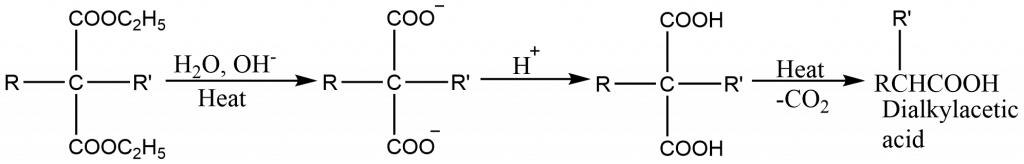
Application of malonic ester synthesis
Some examples of the malonic ester synthesis are:
Synthesis of adipic acid
Adipic acid is dicarboxylic acid. It is obtained from a malonic ester synthesis in which the first step is addition of one mole of ethylene bromide to a large excess of sodiomalonic ester in alcohol. The excess of ester leads to displacement of both bromines from ethylene bromide and gives a tetraester. Hydrolysis and decarboxylation of the tetraester yields the adipic acid.
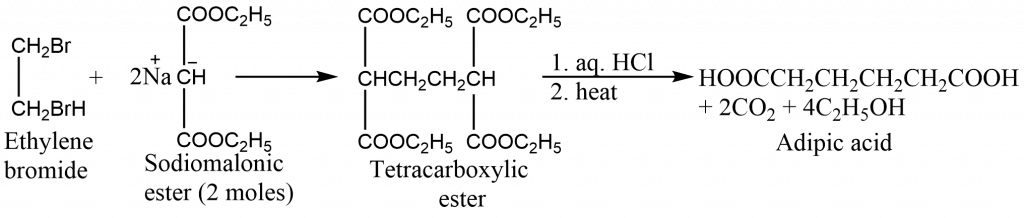
Synthesis of Ketones
Ketones may be prepared from a malonic ester by treating the sodiomalonic ester with an acid chloride, followed by hydrolysis and decarboxylation. The reaction is generally carried out in polar aprotic solvents like dimethylsulphoxide (DMSO) or dimethylformamide (DMF), as the acylating reagents react with alcohols. Malonic ester is treated with sodium hydride in an aprotic solvent to obtain sodiomalonic ester.
For example, 3-methyl -2 -pentanone may be synthesized as described below.
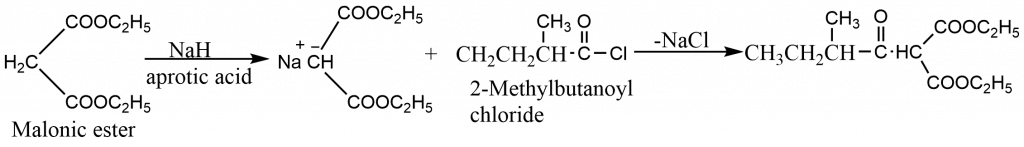
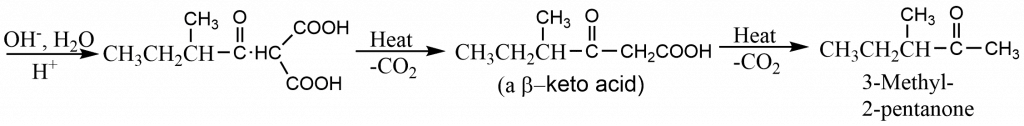
References
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987






