Table of Contents
ToggleHell volhard zelinsky reaction, examples, mechanism, and applications have been discussed here. This reaction was first of all reported by Hell in 1881, and later improved by Volhard and Zelinsky in1887, thus known as hell volhard zelinsky reaction.
Hell volhard zelinsky reaction
Hell Volhard Zelinsky is a reaction between aliphatic acids having at least one alpha-hydrogen and bromine in presence of phosphorus and phosphorus tribromide to give alpha-bromoacid. Simply, alpha-halogenation of carboxylic acid by using X2 and PX3 is known as hell volhard zelinsky reaction.
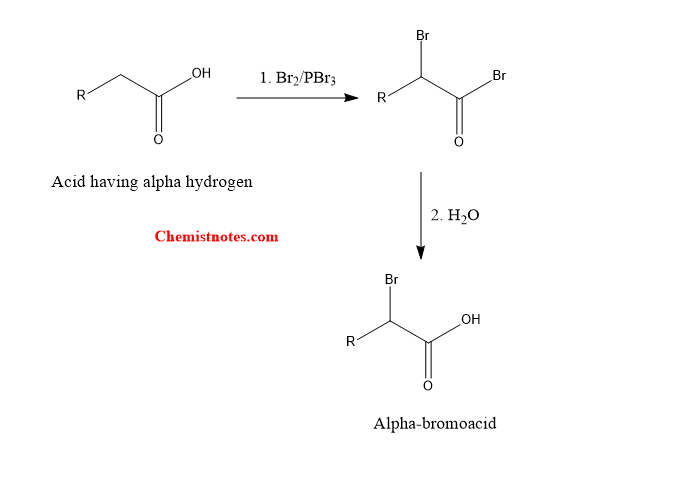
This reaction is a type of substitution reaction in which alpha hydrogen of an acid is substituted by bromine atom to give bromoacid.
Requirement:
- hell volhard reaction is not given by those acid which do not have alpha hydrogen. It means, hell volhard reaction is given by acid having at least one alpha hydrogen.
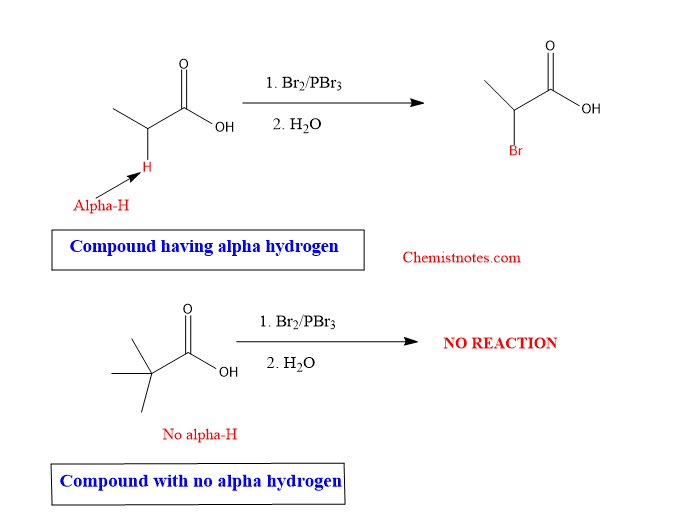
If the reaction is carried out for the chlorination of carboxylic acid, then the reaction is known as Hell volhard zelinsky chlorination. The carboxylic acid must have at least one alpha hydrogen.
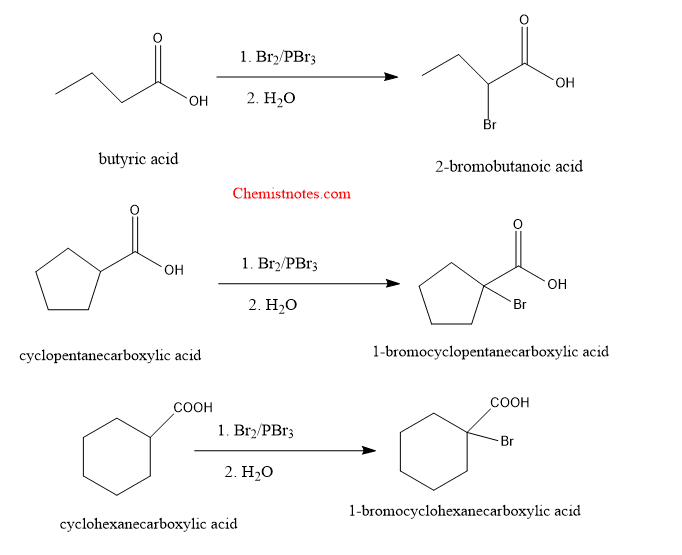
Examples of Hell volhard zelinsky reaction:
Let’s see some examples of these reactions:
Hell volhard zelinsky reaction mechanism:
The carboxylic acid interacts with PBr3 first, forming an acid halide that is in equilibrium with an enol. This enol then acts as a nucleophile, causing halogenation at the site. Hydrolysis, in the end, regenerates the carboxylic acid. Let’s see how the mechanism of reaction proceeds.
Step 1: The double bond of the C=O group of acid attacks the phosphorus atom of PBr3.
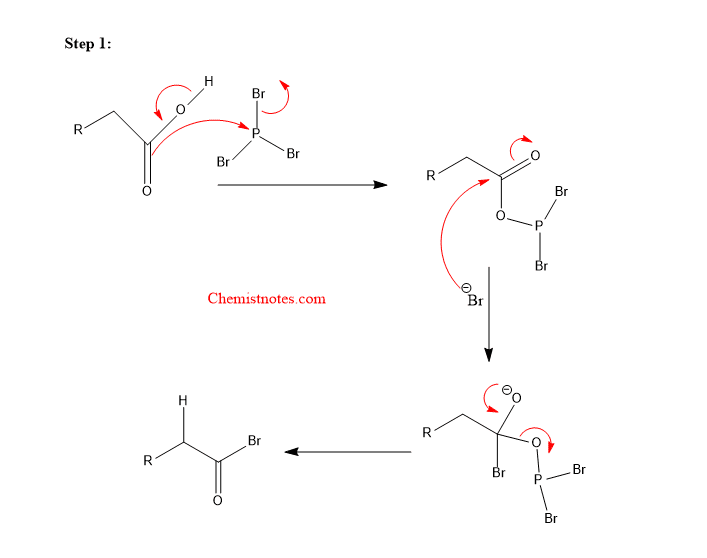
Step 2: The Br– ion abstracts alpha hydrogen from the bromoketone.
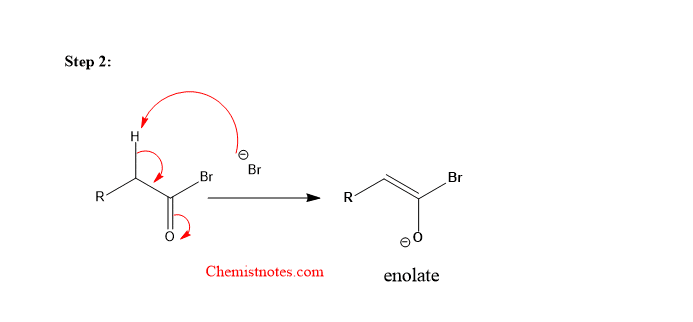
Step 3: The enolate formed reacts with the Br2 molecule.
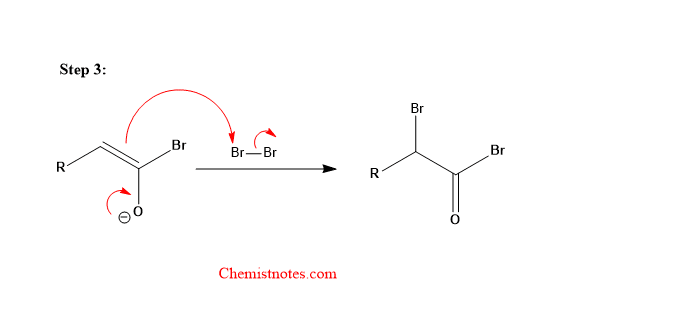
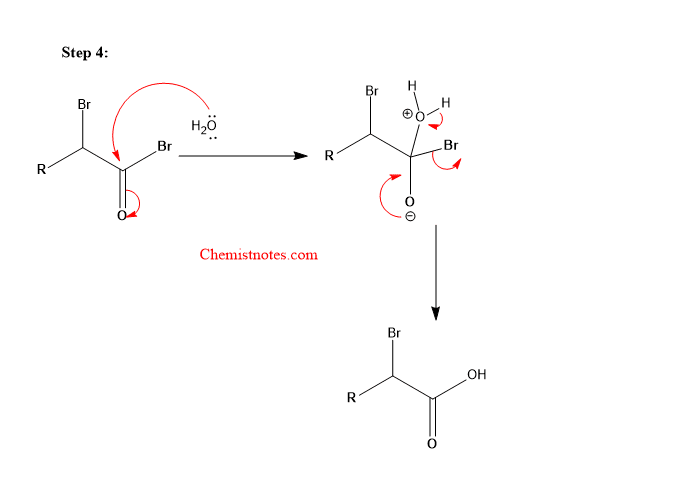
Step 4: Hydrolysis
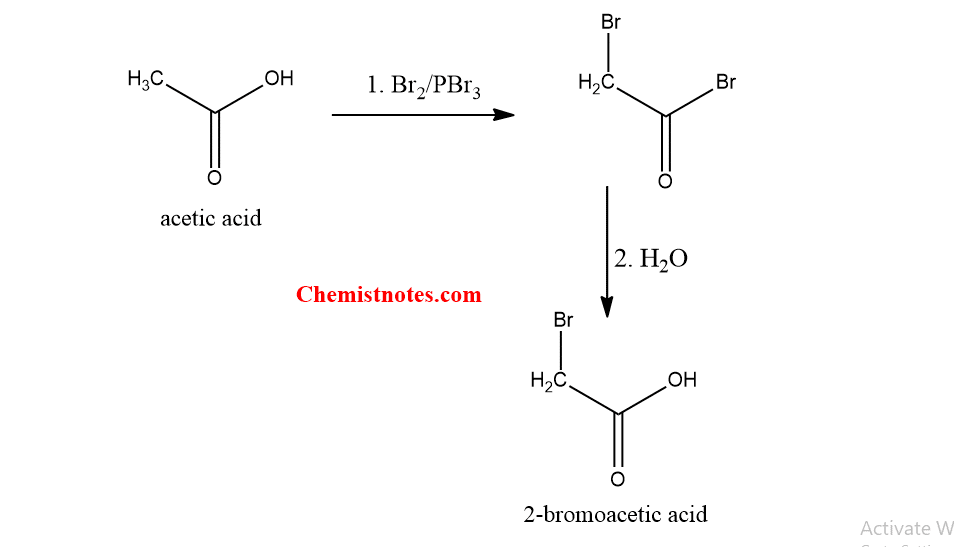
Hell volhard zelinsky reaction of acetic acid
Acetic acid or ethanoic acid can undergo this reaction because it has hydrogen at alpha-carbon. Acetic acid on treatment with Br2 in presence of phosphorus tribromide.
Application of Hell volhard zelinksy reaction
Hell volhard zelinsky reaction is a very important synthetic reaction. Some of the important synthetic applications are listed below:
A. Synthesis of Malonic ester: Malonic acid can be synthesized by hydrolyzing the alpha cyano acid, which is prepared by treating alpha Bromoacid with cyanide ion.
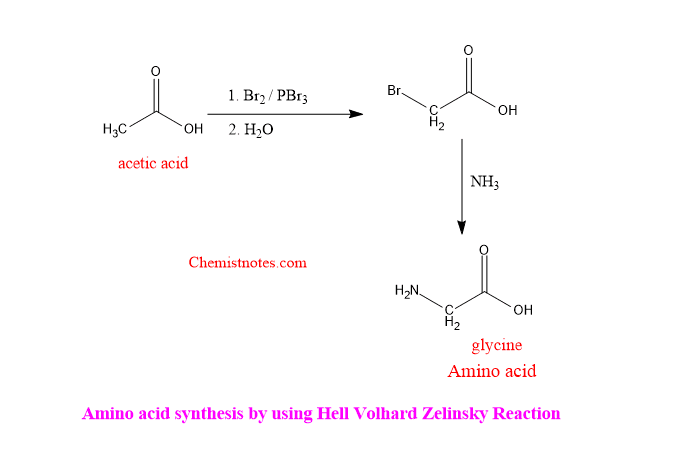
B. Amino acid synthesis by hell volhard zelinsky: Hell volhard zelinsky reaction can be used to synthesize amino acids. The formed alpha-Bromo acid is treated with excess ammonia to obtain amino acid. One of the examples of it is shown below:
References:
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987
Please comment if you find any mistakes in this post. If you want to learn about Wittig rearrangement, click here.
what is hell volhard zelinsky reaction?
Hell Volhard Zelinsky is a reaction between aliphatic acids having at least one alpha-hydrogen and bromine in presence of phosphorus and phosphorus tribromide to give alpha-bromoacid.






