Table of Contents
ToggleDieckmann condensation mechanism, examples, and applications in organic chemistry have been discussed here
Dieckmann condensation definition
Dieckmann condensation is an intramolecular claisen condensation in which a cyclic ketone derivative is formed when an ester of C6, C7, or C8 dibasic acid undergoes intramolecular condensation in presence of condensing agents such as sodium, sodium ethoxide, or potassium t-butoxide. In other words, it is base-catalyzed intramolecular condensation of diester that leads to the formation of 5- or 6-membered cyclic β-keto esters.
Dieckmann condensation examples
Some of the examples of Dieckmann condensation reactions are:
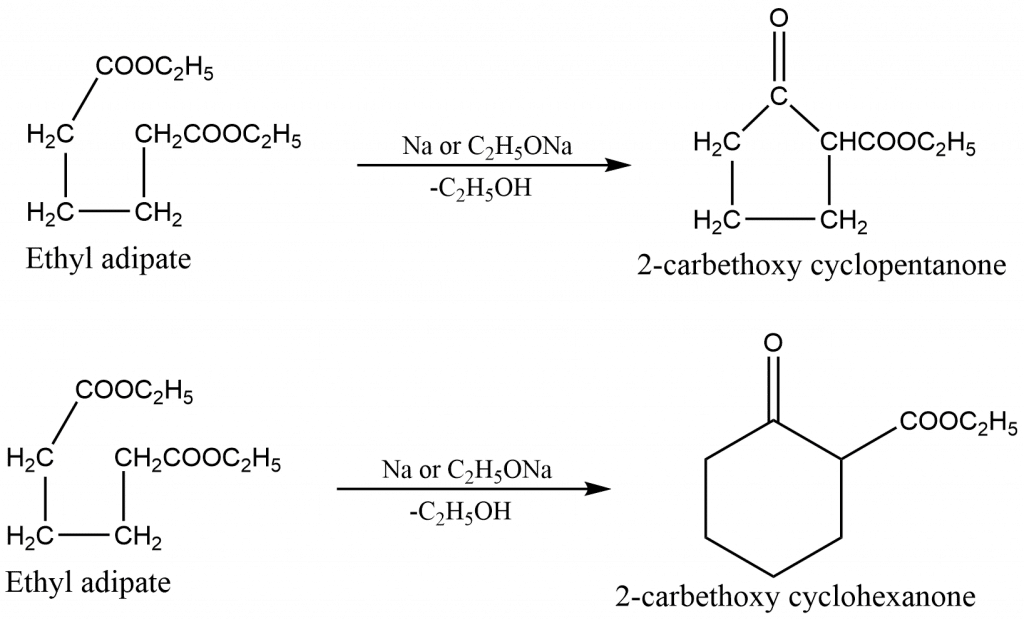
Dieckmann condensation mechanism
The base deprotonates ester at α-position and leads to the formation of carbanion, which then attacks the carbon of the second ester group to generate cyclic enol. Now, protonation leads to the formation of β-keto ester.
The mechanism of Dieckmann condensation can be carried out into multiple steps:

Application of Dieckmann condensation
Dieckmann condensation has been used to form five and six-membered rings in a number of synthesis of natural products such as α-pinene, oestrone, cyclic indole, and so on.
Limitations
- Because of the large strain in the smaller rings formed, esters of dibasic acids with fewer carbon atoms are unable to undergo Dieckmann condensation.
- Since the intramolecular reaction becomes smaller as the chain length increases and intermolecular condensation reaction is favored, esters of dibasic acids with more than eight carbon atoms do not undergo Dieckmann reaction..
Dieckmann condensation video
MCQs/FAQs
Dieckmann condensation reagent
The condensing agent for Dieckmann reactions is sodium, sodium ethoxide, or potassium t-butoxide.
Dieckmann condensation problems
Esters of dibasic acids having fewer carbon atoms and carbon atoms greater than eight, unable to undergo intramolecular condensation reaction to form β-ketoester is one of the major problems of Dieckmann condensation reactions.
Dieckmann condensation reaction
Dieckmann condensation is an intramolecular condensation reaction of a diester with a base leading to the formation of β-ketoester.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
- Janice Gorzynski Smith (2007). Organic Chemistry (2nd ed.). pp. 932–933. ISBN 978-0073327495.






