Table of Contents
ToggleDEPT spectra is very useful spectra in 13C NMR spectroscopy which is used to determine the number of protons(hydrogen) directly attached to individual carbon atoms present in the molecule. The DEPT is the abbreviated form of Distortionless Enhancement by Polarization Transfer.
In the DEPT technique, the sample is irradiated with a complex sequence of pulses in both the 13C and 1H channels. The DEPT pulse sequences of variable proton pulse angle theta are set, the proton pulse angle 90 is set for one subspectrum which is known as DEPT 90, and angle135 is set for other separate experiments, known as DEPT 135. The pulse angle of 45 can also be applied during the experiment, which usually contains up-phased( positive) peaks of all types of carbons except quarternary carbons. This is known as DEPT 45.
This experiment can be done in a reasonable time and on small samples, and it is several times more sensitive than the ordinary 13C experiments.
DEPT Spectra
A DEPT spectrum consists of the following subspectrum.
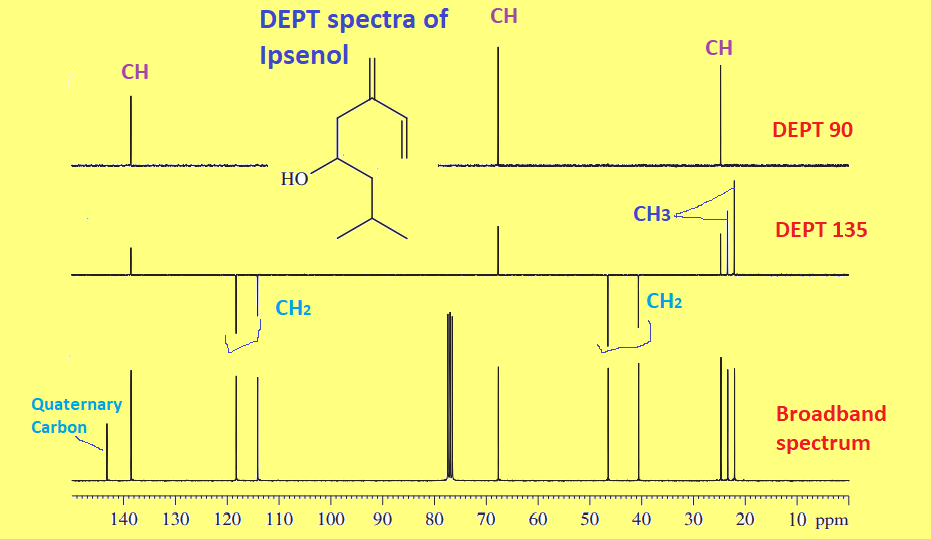
- The main spectrum is actually a broadband spectrum(1H-decoupled 13C spectrum) where CH3, CH2, CH, and quaternary carbon are observed.
- The middle spectrum is DEPT 135, where the peaks of CH3, CH2, and CH are obtained but the peaks of quaternary carbon are not obtained. CH3‘s and CH’s peaks are phased up whereas that of CH2‘s are phased down.
- The last spectrum is DEPT 90, where only CH carbons are detected.
DEPT spectra interpretation
For the interpretation of DEPT Spectrum, there may be many more ways but the easiest one has been discussed below in a stepwise manner.
Step 1: First of all, look for the peaks that are in the broadband spectrum( 1H-decoupled 13C spectrum) but absent in both DEPT 135 and DEPT 90 spectra.
If there are peaks present in the broadband spectrum and absent in DEPT 135 AND DEPT 90 spectra, these peaks are of quaternary carbon( carbon with no proton attached). It means the quaternary carbon peaks are only present in the broadband spectrum. The intensity of such a peak may not be so intense but can be easily identified by comparison with the other two DEPT spectra.
Step 2: Look at DEPT 135 spectra and identify phased-down peak
If there are inverted peaks or down-phased(Negative) peaks present in the DEPT 135 spectrum, these are easily identified as the peak of CH2, because the peaks of CH3 and CH are phased up( Positive) and only the peak of CH2 is down-phased.
Step 3: Compare DEPT 135 and DEPT 90 spectra
We have already identified quaternary carbon and CH2 peaks above in steps 1 and 2. Now, let’s identify CH3 and CH peaks by comparing DEPT 135 and DEPT 90 spectra. The DEPT 90 spectrum contains only CH carbons peak but not other carbon peaks. Hence, those up-phased peaks in DEPT 135 and absent in DEPT 90 are identified as CH3 carbon peaks.
In this way, DEPT spectra can be interpreted to identify all the types of carbon on the basis of different phases in spectra. The number of each type of carbon is also determined by counting the number of peaks in the spectrum.
DEPT spectra examples
The above three steps of interpretation can be now applied to the following DEPT spectra of Ipsenol.
FAQs/MCQs:
What is the full form of DEPT?
The full form of DEPT is Distortionless Enhancement by Polarization Transfer.
What does the 90 indicate in DEPT 90?
The 90 indicates the pulse phase angle in DEPT 90.






